EMS Bio and BioLab Fluid Flow
If you’ve ever seen a Houdini movie you’ll know something immediately about a BioLab Fluid Flow sales seminar just put on by EMS Bio. It’s worth reviewing below just to see how they create an impossible escape from a place that’s dangerous, just like Harry Houdini did way back when. Let’s dig in.
The World’s Greatest Escape Artist-Harry Houdini
Harry Houdini was a turn of the 19th-century escape artist with a famous trick called the Water Torture:
Harry would have his feet chained and shackled to the top of a box. That glass box was filled with water and Hary would have himself lowered into it. The band and announcer would count down the minutes. After the first minute had passed, Harry would uncomfortably squirm like he was in serious trouble and just then they would lower a curtain over the tank. The crowd would be howling as 4 or 5 minutes passed without Houdini showing up. Then the drape would be removed showing Houdini standing safely on top of the water torture box.
How did he do it? With deception and a trick. His wife, who was his helper, would kiss him goodbye before chaining him up and would pass the key to the locks and shackles in that kiss.
EMS Bio is a distributor for the amniotic product called BioLab Fluid Flow and just pulled off a similar Houdini-like trick. IMHO, They seemingly managed to escape from ALMOST certain medicare fraud. How did they do it? Like Houdini, with an ample helping of trickery. Let me explain.
Medicare, Amniotic Fluid and Q-Codes
We have a few companies out there claiming that Medicare will cover amniotic fluid injections for things like back pain or knee arthritis. These companies offer up as proof a Q-code. What’s that? A billing code for getting products reimbursed. The problem? It’s a trick. See my video to find out more:
The World’s Second Greatest Escape Artist?The EMS Bio Presentation
A colleague recently sent me a sales presentation hosted by EMS Bio for BioLab Fluid Flow, which they are selling to providers. It’s quite a Houdini escape trick. Let’s dive in.
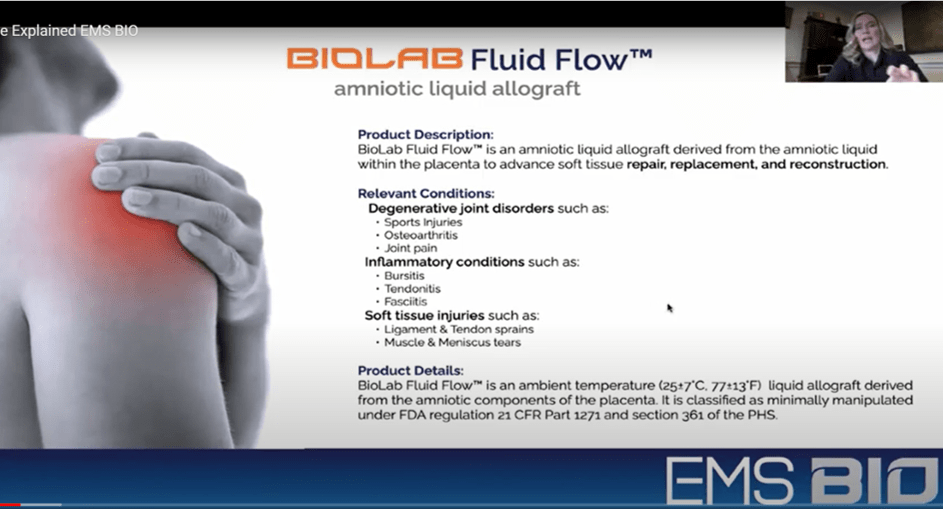
The salesperson begins with this slide:
Problems? This product is only registered with FDA as a 361 tissue. This is a quick 45-minute online affair without any FDA review of the product. Hence, treatment claims are NOT permitted. If you want those, then you need to get the product through years of clinical trials and have those reviewed by FDA. BioLab has not done this, so IMHO, these treatment claims of the product’s use in degenerative joints, inflammation, and soft-tissue injuries are NOT FDA compliant.
IMHO, the slide above is where this presentation really comes off the rails with our first bit of stage trcikery. That first sentence is nuts, “The vast majority of all amniotic products are NOT covered by Medicare because they contain stem cells. Stem cells are considered experimental”. Having reviewed the Medicare Q-code granting sessions, they were rubber-stamp meetings until recently when FDA got involved. Now, the agency has shut down all new Q-codes being granted for birth tissues. Why? Not because they contain stem cells, but because 361 tissue manufacturers are not permitted to make claims like those made here for Fluid Flow. Want proof that making any of these claims for an amniotic fluid product is not Kosher? See here, here, and here. Hence, Medicare is now under an FDA veto on new products getting Q-codes.
This slide is also made for the gullible. The indications and use for a product is determined by FDA, NOT Medicare. So what you see for BioLab Sciences Fluid Flow and the others here are product descriptions submitted by the manufacturer in a code granting session that got inadvertently rubber-stamped by Medicare while FDA was asleep at the switch. NONE of these claims have been approved and reviewed by FDA, which has the final say on product claims. Nor are any of these claims permitted for any 361 registered tissue. That’s why we see the bevy of Warning and Untitled letters sent to amniotic fluid manufacturers making similar claims.
While the salesperson told us above that the reason stem cells don’t have Medicare coverage is that they’re considered experimental, here we find out that Fluid Flow as a product is also considered experimental by Medicare. Huh? She also claims that some Medicare regions aren’t covering Fluid Flow in their fee schedules, but those regions just haven’t gotten the memo yet.
Now, this is where the big magic trick begins. One of the issues with any birth tissue product when used for things like knee arthritis, soft-tissue injuries, and back pain is that regardless of which reimbursement code got assigned a number there must be scientific and clinical review by a Medicare committee of that treatment for every medical indication. That generates what’s called an LCD (Local Coverage Determination), which is the guideline that would tell us whether Fluid Flow is covered to treat things like knee arthritis, soft-tissue injuries, and back pain. Is there any LCD that shows that Medicare reviewed the clinical research and that Fluid Flow is covered for these things? Nope.
Using our analogy, this is the point that Harry Houdini is hanging upside down and underwater and looking stressed as one-minute passes and they lower the curtain so you can’t see what’s happening. At that moment, Harry takes the key out of his mouth and unlocks his shackles and uses the trick latch to easily let himself out of the water tank while the audience is being worked up by the MC, believing that poor old Harry is still hanging upside down in the tank.
So what’s the trick key here? The 21st Century Cures Act! This was a seminal piece of legislation that created a fast track for FDA approval of regenerative medicine products, but the salesperson will use it here as the magic that seemingly allows Medicare to cover her product.
This slide states that LCDs do not determine coverage since changes were made with the 21st Century Cures Act. This one had me going for a second until I Googled what changes happened. It’s also jargon-rich, so this is that point where Houdini tricks the audience because nobody will take the time to look this stuff up
What’s going on here? The claim is that LCDs no longer matter and it’s all about the Medicare Coverage Database. Then the concept of “Articles” is brought in to further confuse the issue.
At face value, this means that a rubber stamp session to get assigned a billing code, where no scientific analysis of whether a product is effective takes precedence over physicians and scientists meeting to review the medical literature on a treatment. Yeah, right. If you believe that I have some swampland to sell you in Florida, I just need to print up some deeds real quick.
What does Medicare say about all of this? Nothing like what’s described. Here’s a summary of the changes made to the LCD process due to the 21st Century Cures Act. Those changes are focused on cleaning up the LCD process, streamlining it, and making it more transparent. The other thing that happened was that the 21st Century Cures act moved billing and diagnosis codes out of the LCDs and into articles. This did not have the impact of getting rid of LCDs or making them less important as EMS Bio claims.
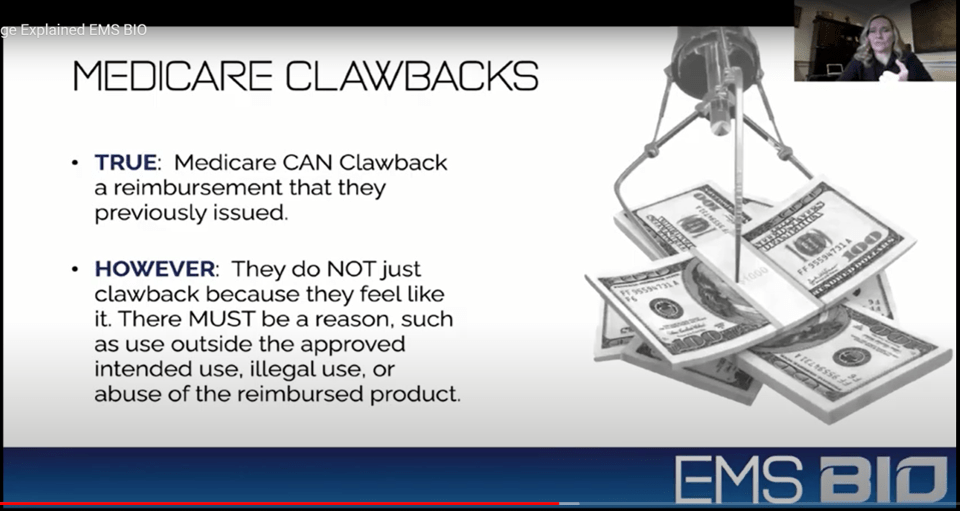
Now here’s a slide that everyone should pay attention to. Medicare is an electronic payment system. Hence, if something is set up in their system a certain way and the right combination of codes are submitted, Medicare will pay even if they shouldn’t pay that claim according to the LCD. The responsibility for knowing whether it’s a legal or illegal claim is placed on the doctor’s office. Hence, once Medicare discovers its error, they send a letter asking for the money back, which is called a “Clawback”.
The problem for doctors is that given that Fluid Flow and other birth tissue products are reimbursed to the tune of 4-16K per patient, these numbers get quite big quite fast. In fact, EMS Bio tells us that we can inject this stuff up to three times a year into the same knee joint! Hence, if we treated 10 patients a week at an average of 10K per patient for indications like knee arthritis, soft-tissue injuries, and back pain for a year, we would owe Medicare back 5 million dollars! This one clawback would bankrupt almost all smaller or medium-sized clinics.
The upshot? As you can see, like any great magic escape or trick, there is always that moment when you get the audience to bite. Where they believe that you’re drowning in a glass water tank rather than having a wet, but comfortable smoke on top of it. IMHO, the EMS Bio presentation is just like that, as once you know the tricks, you can never watch it quite the same way again.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.