Disc Injections With Platelet Raisins and Protein Syrup

The last blog I published was on the mounting evidence for using PRP to treat the common causes of spinal pain. However, in that literature review, one study stood out. That research was abruptly halted at eight weeks, which was curious. As I sat down to read and analyze this paper in more detail, it got stranger because the authors never used PRP, but instead, platelet raisins plus protein syrup. Let’s dig in.
PRP Research
The good news is that there are dozens of randomized controlled trials that have used PRP and have shown superior results to placebo or other common injectates. That’s positive not only because those results show superiority but also because these data fit with the outcomes generated by physicians who use this treatment every day. However, there are a few negative studies that tend to skew the perception of PRP as a treatment.
I’ve covered a few of the negative results that didn’t agree with the majority of the research. Some never actually used PRP and instead used a plasma with too few platelets to meet the definition for that substance. Given that this definition was established decades ago, it’s a fair question to ask why the study designers never realized they were spending lots of time, money, and resources on a study with such a serious flaw.
The New Platelet Raisins Study-Design and Methods
The new research was performed by Zielinski/Jordan et al. at a few clinical sites and recruited 26 patients who had evidence of discogenic pain (pain coming from the disc), with two patients being assigned to treatment for each one who was injected with saline control (1). The patients were chosen by discography, which is when the doctor pressurizes the disc with contrast to see if it causes pain. The included study participants could have anywhere from 1-4 positive painful disc levels. MRIs also had to show moderate or less degenerative disc disease (Pfirrmann 1-4). Last, the patient had to fail conservative care.
Of note, the exclusion criteria (when a patient couldn’t join the study) allowed for a VERY HIGH amount of opioid use. The max opioids allowed were up to 180 mg oral morphine a day equivalent. That’s about 120 mg of oxycodone or two 60mg Oxycontin tabs per day or twenty-four 5mg Percocet tabs a day! Regrettably, we never learned how much narcotics the study participants were using or if that changed during the study.
Another concern for the study design and methods is that all patients were UNBLINDED at eight weeks! Meaning that the comparative study results would become invalid after that time. More on that below.
Was This a PRP Study?
The good news is that the study authors actually used decently concentrated leukocyte-poor PRP by using the Emcyte kit. The bad news is that they then went further, bringing what was injected well out of the realm of what is normally considered PRP. Let’s dig in on that below.
Here’s what was created for injection:
- 53 ml of whole peripheral blood was double-spun, oddly, in two different centrifuges. This created a platelet and buffy coat pellet.
- They then removed all of the platelet-poor plasma (PPP) but 2 ml. Assuming a platelet recovery of about 60-70%, at this point, they have highly concentrated PRP.
- They then took 25-30 ml of PPP which was likely leukocyte rich, and used a “protein concentrator.” That’s a euphemism for a device that dehydrates the plasma by pulling water out, concentrating proteins and anything else in the sample. This was reduced in volume many times so that 4ml of solution was output. Or said another way, it was a protein syrup.
- They then took the highly concentrated PRP and diluted it 1:2 with the protein syrup. The end result was moderately concentrated “PRP” plus the protein syrup made in step 3. However, that injectate likely wasn’t still PRP, as I’ll explain further below.
The “Futility Analysis” Was Indeed Futile
Our fearless study investigators, after injecting whatever the heck PRP+protein syrup is called into painful discs, had a cross-over point at eight weeks. Again, that meant that this was the point where people who didn’t get a real treatment could “cross over” to get their discs injected with “PRP+protein syrup.” At this point, it looks like the authors decided to perform a “futility analysis.” This is where some preliminary stats are run to determine if the study should continue. This is usually utilized when the study has a life-threatening endpoint or to see if the results are so good that it’s no longer ethical to keep sick people from a miracle cure. This is not usually done to end a low-risk study where people may still respond at a later date and standard-of-care options like low back surgery are plentiful.
To understand why performing a futility analysis at eight weeks makes no sense, let’s review a smattering of PRP studies that were published earlier.
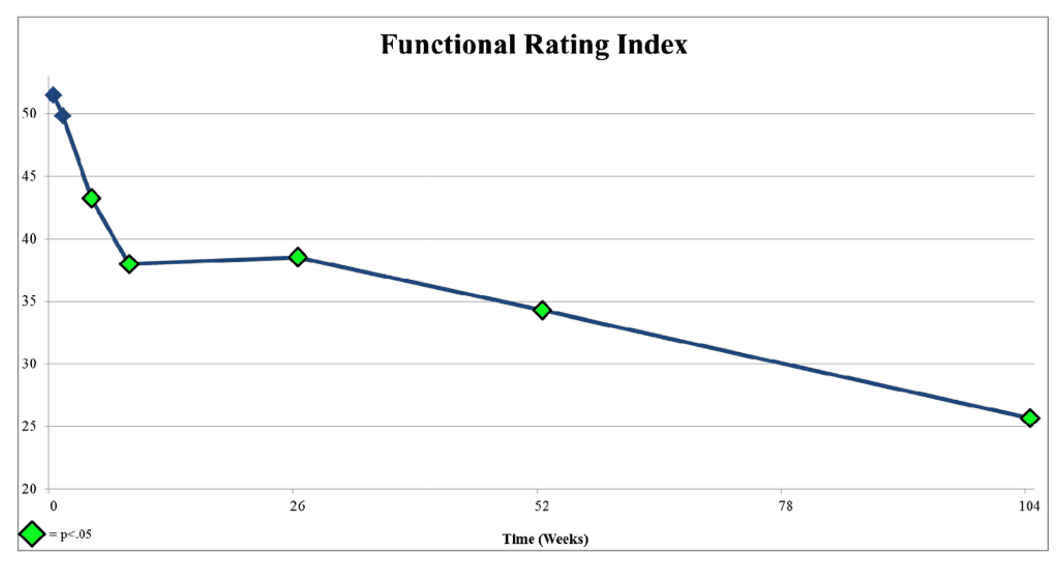
Above is a graph from the Lutz et al. paper in 2016 that launched this new PRP disc injection study (2). There are two big inflection points here in function. The first is at around 6-10 weeks, where function improves, and then from 6 months on, the function improves yet another 50%. We know this because, unlike the PRP+protein syrup study, the Lutz PRP study didn’t end the study at eight weeks and continued to collect data.
Now let’s look at graphs from other papers.
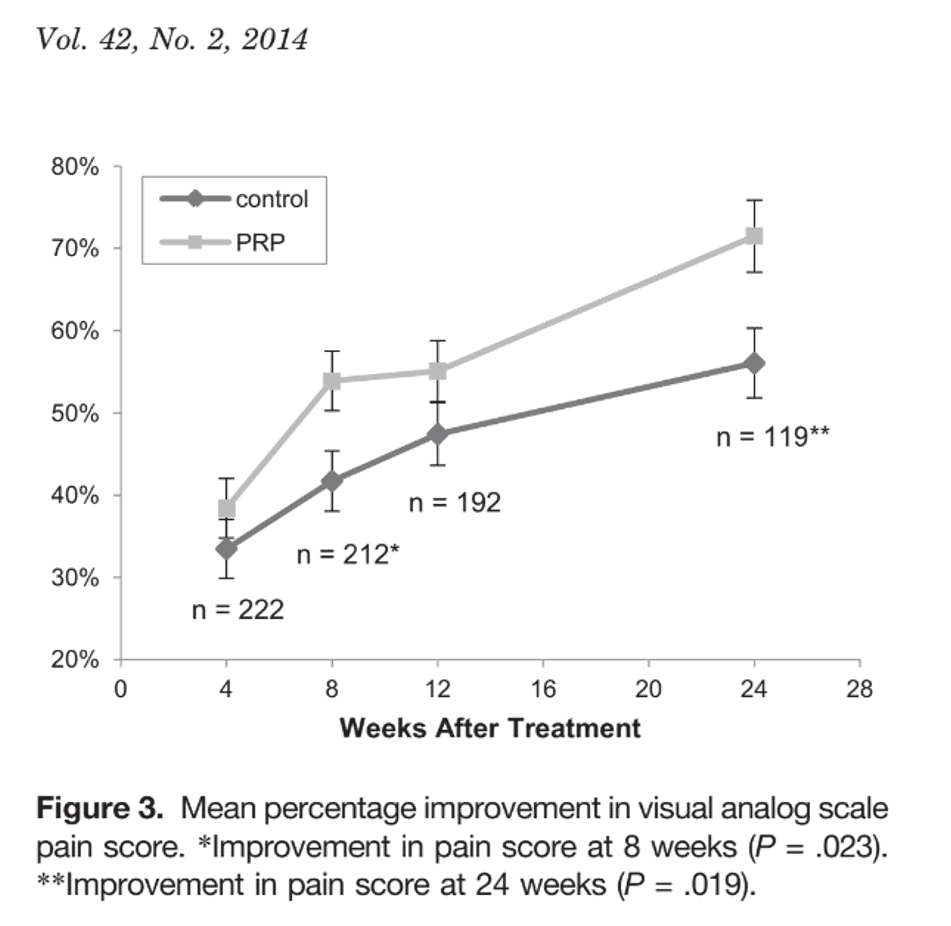
This is from Mishra et al. on using PRP to treat epicondylitis (3). Note that while there is some improvement at eight weeks, most of the improvement is after that time.
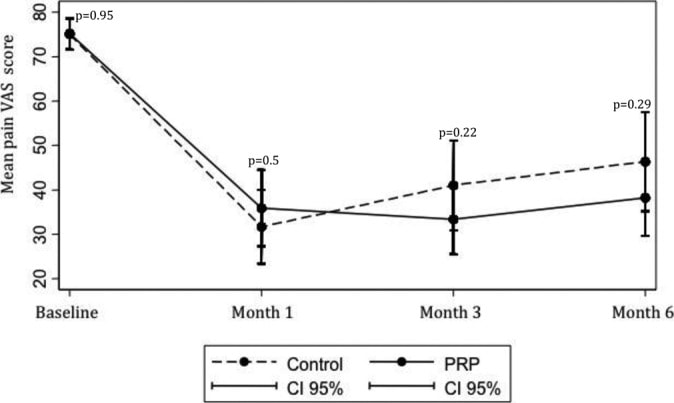
Check out this graph above from a study by Jubert and Navarro et al. on PRP used to treat knee arthritis (4). Note that the cross-over point where PRP starts to perform better than saline control is about eight weeks. Meaning if you had ended the study there, the story told by the data would have indicated that PRP worked no better than saline. However, had you continued the study for just another month, the story becomes that PRP works better than saline.
You get the point. Ending data collection at 8 weeks, based on what others have reported, is not a wise nor educated decision.
PRP vs. [PRP+Protein Syrup]
The positive RCT on PRP injection for lumbar disc pain by Lutz et al. used a leukocyte-rich, moderate-concentration PRP. However, the Zielinski et al. study used moderate concentration PRP plus protein syrup. Meaning the latter study didn’t test the same substance as the former. Let’s explore that a bit further.
What’s in protein syrup? The Zielinski study used the BioRich Medical ProPlaz Protein Plasma Concentrator. To date, there is zero clinical data that I can find published on the use of the output of this machine. What does Biorich say it produces? It says nothing on its website. I reached out to an experienced device industry insider and confirmed that the system is a dehydrator. That presents new problems, as we’ll see below.
Platelet Raisins
We know that the Biorich Proplaz dehydrates the plasma sample or, said another way, it pulls out the water. The idea behind that type of device is that it may be able to concentrate helpful proteins in the sample, like A2M. However, when combined with PRP, it has the potential to create another problem.
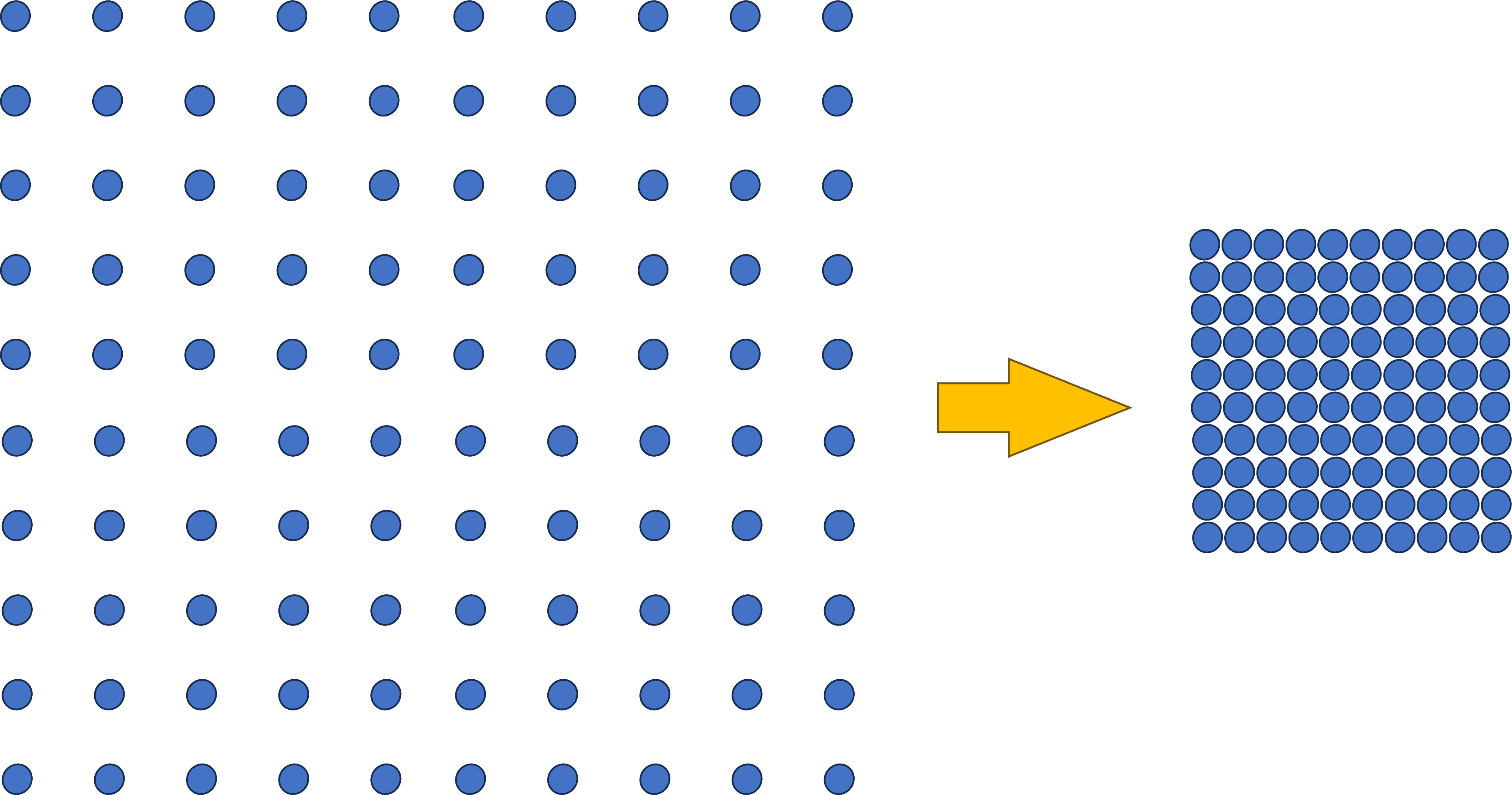
To better understand that issue, note that below I have 100 balls spaced apart to represent a specific protein concentration. We know that the Biorich Proplaz here removed enough water so that about 30 ml went to 4 ml. Meaning we concentrated the proteins about seven times. Hence to represent that concentration, the balls on the right are now much closer.
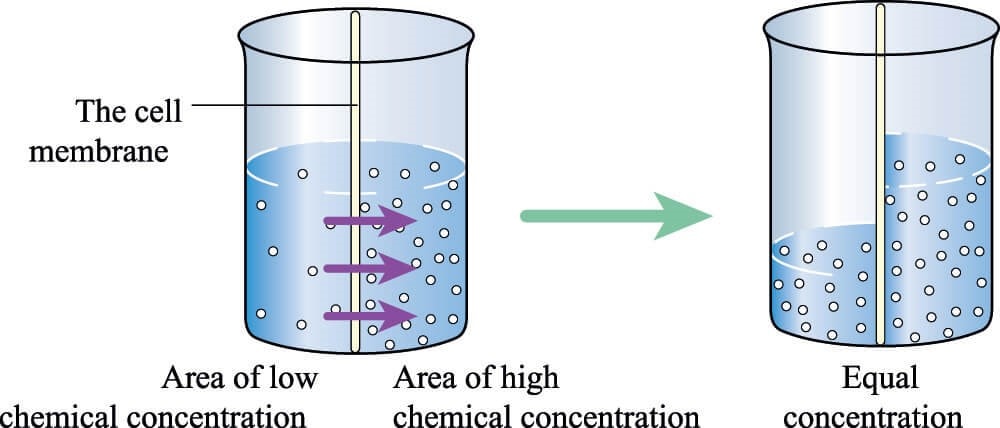
Why could this be a problem with platelets? We know from basic chemistry that when there is a membrane involved and two solutions with different concentrations, water flows from the less concentrated solution to the more concentrated one until the concentrations equilibrate across the membrane.
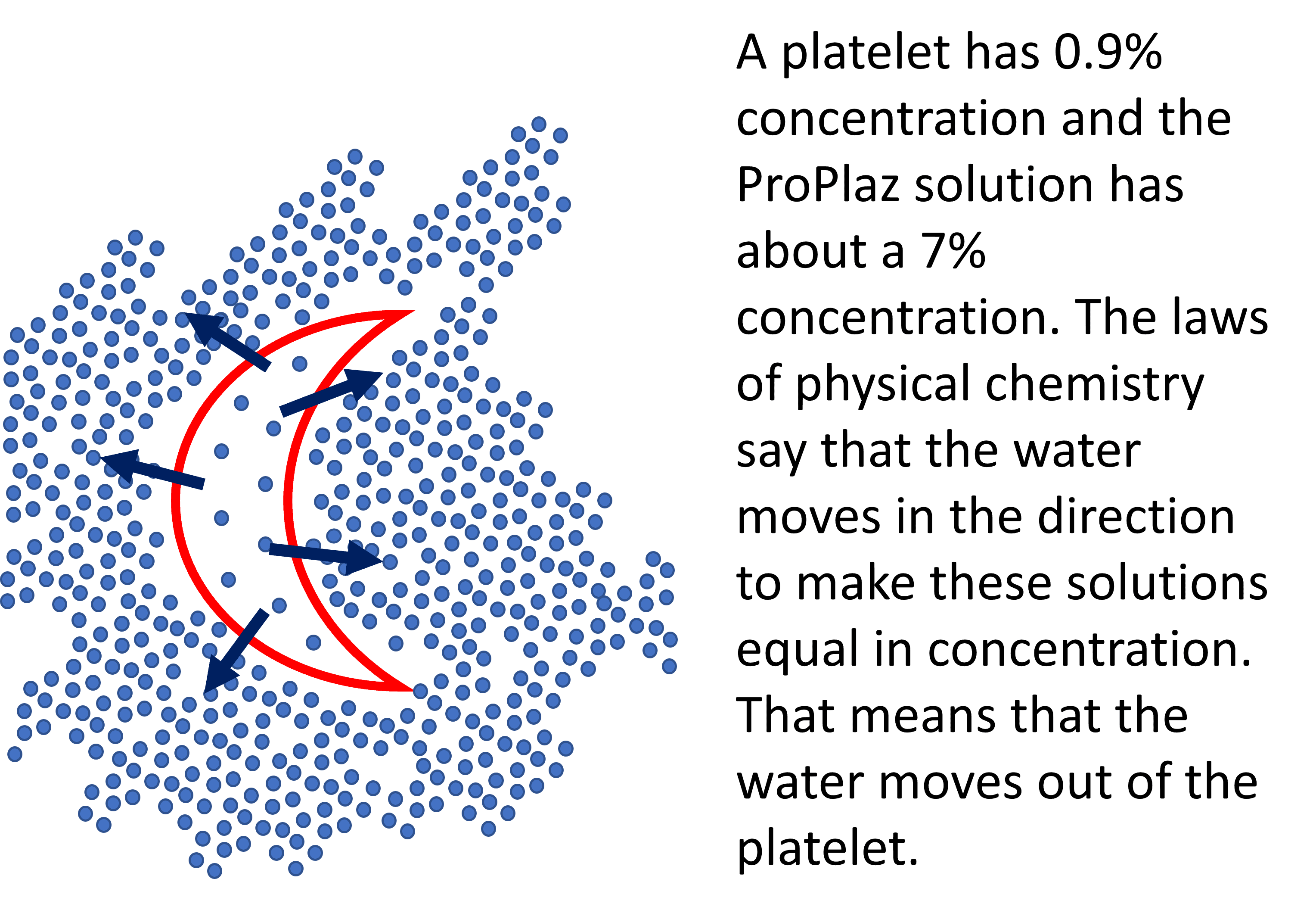
Take, for example, a platelet that has a concentration of 0.9% like the rest of the body. Once we surround that platelet with our 7% concentrated plasma syrup created by the Biorich ProPlaz, we have the same situation as the chemical concentration gradient shown above. The water will flow out of our platelet and into the surrounding solution.
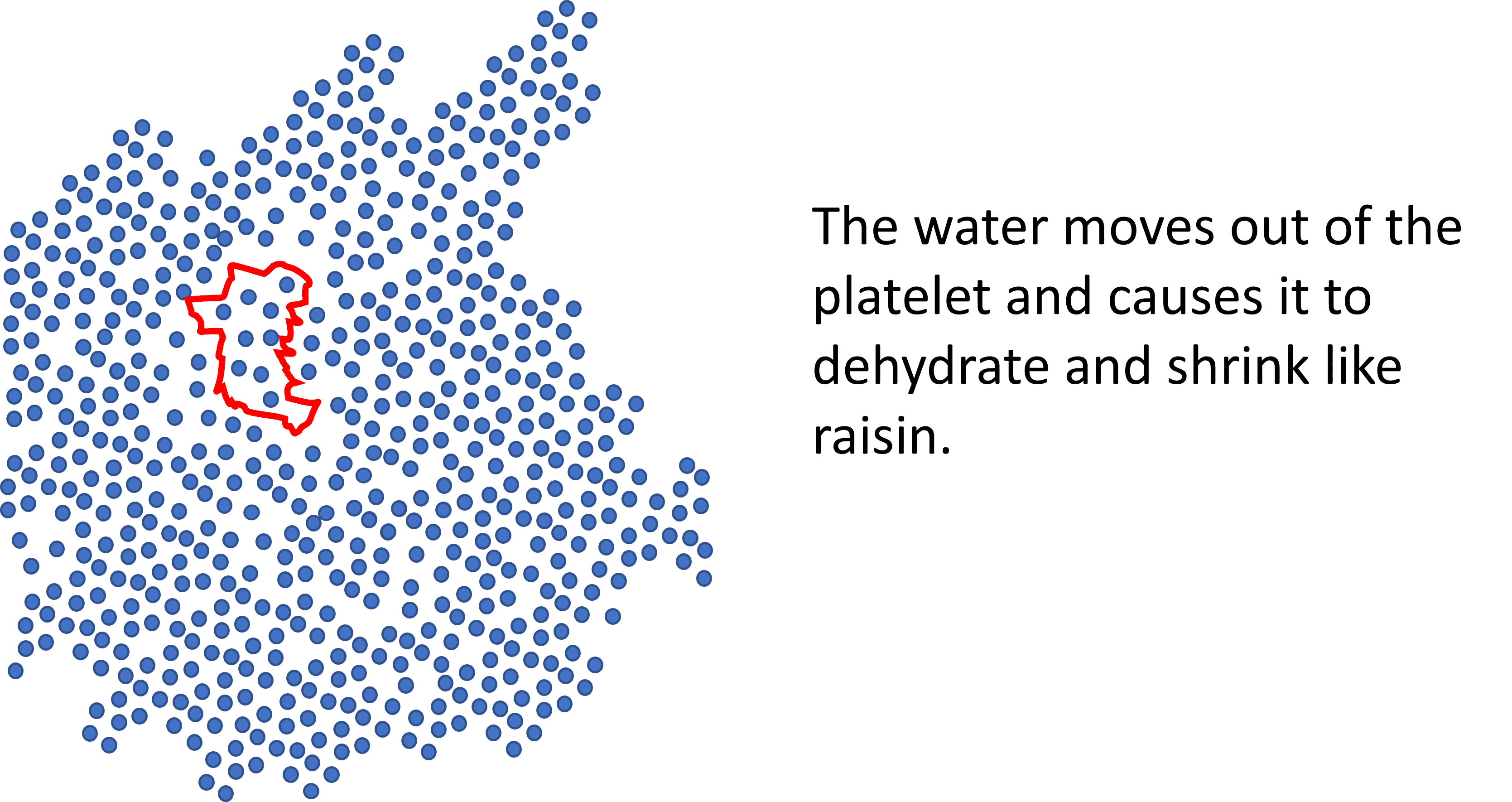
What do we get when we dehydrate a platelet? A platelet raisin. Given that PRP depends on platelets degranulating growth factors and exosomes, how this impacts the functioning of a platelet is anybody’s guess. If I had to bet, I would wager that a platelet raisin does not function like a normal platelet.
Summary
We have a research study that ended way too soon. Based on that, it’s a fair question to ask “Why”? We also know that the investigators claimed to use PRP but never used PRP but instead injected platelet raisins plus protein syrup. We can also postulate that by combining the dehydrated plasma syrup with platelets, it’s likely that the function of the platelets was degraded. All of this likely explains the experimental differences between this study and the Lutz publication.
The upshot? Does injecting platelet raisins plus protein syrup work to help disc pain? Who knows, as this study ended before it began. I do know that this study didn’t test PRP and never should have been labeled as such. Hence, I will be omitting it and placing a big asterisk on it in my previously published infographic so it can be interpreted in context.
_________________________________________________
References:
(1) Zielinski MA, Evans NE, Bae H, Kamrava E, Calodney A, Remley K, Benyamin R, Franc D, Peterson MR, Lovine J, Barrows HR, Mahdavi K, Kuhn TP, Jordan S. Safety and Efficacy of Platelet Rich Plasma for Treatment of Lumbar Discogenic Pain: A Prospective, Multicenter, Randomized, Double-blind Study. Pain Physician. 2022 Jan;25(1):29-34. PMID: 35051141.
(2) Monfett M, Harrison J, Boachie-Adjei K, Lutz G. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: an update. Int Orthop. 2016 Jun;40(6):1321-8. doi: 10.1007/s00264-016-3178-3. Epub 2016 Apr 12. PMID: 27073034.
(3) Mishra AK, Skrepnik NV, Edwards SG, Jones GL, Sampson S, Vermillion DA, Ramsey ML, Karli DC, Rettig AC. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014 Feb;42(2):463-71. doi: 10.1177/0363546513494359. Epub 2013 Jul 3. PMID: 23825183.
(4) Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop J Sports Med. 2017 Feb 13;5(2):2325967116689386. doi: 10.1177/2325967116689386. PMID: 28255569; PMCID: PMC5315239.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
