How Are Companies Getting Paid for Amnion? The Magic Trick Explained
If you’re following the Medicare reimbursement scam for amnion and umbilical cord products I’ve been covering, you may wonder how that scam began. While I knew some of this story prior to yesterday, I still hadn’t put all of the pieces of the fraud together to show a clear picture from start to finish. Now I know enough to show you how the scam begins and evidence that both CMS and FDA are beginning to catch on, which is very bad for the scammers.
The Medicare Q Code Scam
First, under no circumstances, based on Medicare guidelines should amniotic or umbilical cord tissue injections be covered for orthopedic or pain conditions. Period, do not pass Go. There is no ambiguity here based on all of the Medicare guidelines I have reviewed. Despite that, several shady birth tissue manufacturers have thrown caution to the wind and convinced physicians to bill Medicare for these products. Because of holes in Medicare’s electronic payments system, some claims will even get paid in error. The problem? Knowingly billing Medicare for a non-covered benefit is punishable by 10 years in federal prison per claim submitted.
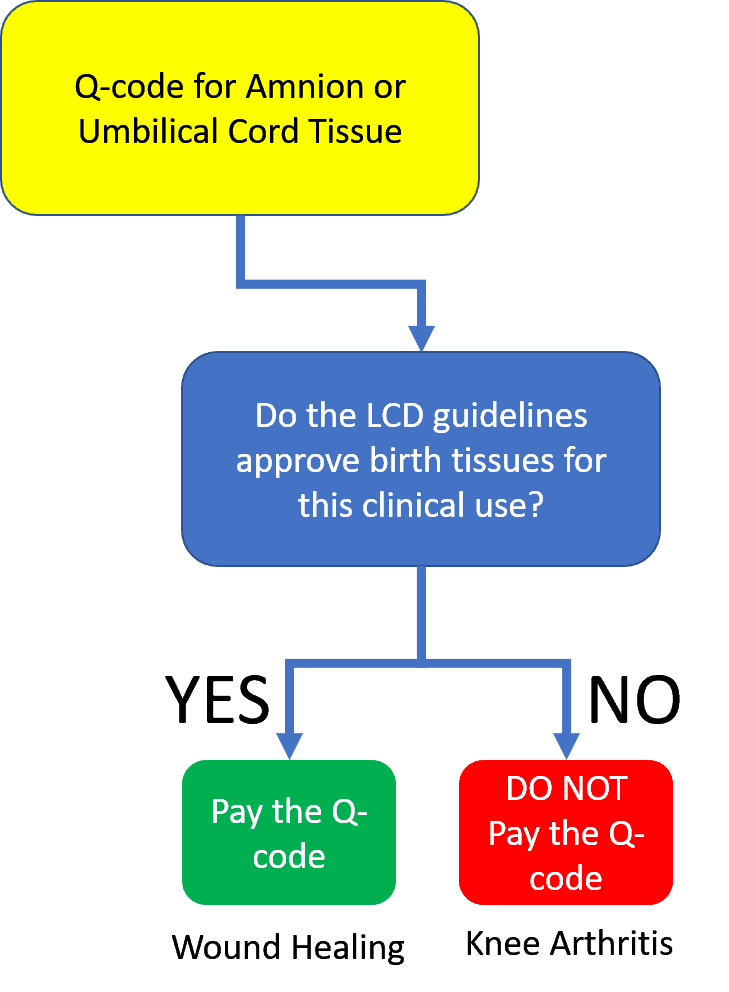
I have diagrammed above how this is supposed to work. As you can see, the Medicare guideline called the “LCD” determines if the claim should be paid. However, to get the scam to happen, like any good magic trick, we need to create a distraction so you never look at that LCD document. Where we want your eyes is on the Q code itself.
So if Medicare clearly doesn’t cover amniotic or umbilical tissue for things like knee arthritis or back pain, how are these claims getting paid? The scam begins with a little known reimbursement concept called a Q code.
The Genesis of the Scam-Applying for a Q Code
A Q code is a HSPCS code, which itself stands for “Healthcare Common Procedure Coding System”. The Q code is created to reimburse a hospital, surgery center, or clinic for a specific drug or biologic product used during a procedure.
How Does a Birth Tissue Manufacturer Get a Q code?
On a quarterly basis, the Centers for Medicare and Medicaid Services puts on a meeting to consider adding new Q-codes. The manufacturer submits a description of its product and what it thinks that product should be used for. This is how our scam begins.
Normally, amniotic tissue manufacturers list medical indications like wound care because that’s all Medicare will cover. However, the critical part of this scam is that you can also insert other language about the clinical uses such as knee osteoarthritis or use in treating tendons, “soft-tissues”, or pain. The more hidden and oblique the better here, because you’re going to use this Q code later to make the billing scam go.
Getting Your Q Code Rubber Stamped
Once you have your description that cleverly disguises your language about using birth tissues for unapproved purposes, you take that application to CMS and hope they never notice. If they don’t, CMS will add your Q code to the long list of other manufacturers that have gotten similar approvals for using amniotic tissue sheets in wound care. However, your product won’t be used that way. Yours will be injected to treat things like arthritis, tendons, muscles, or pain.
The Willy Wonka Golden Ticket of Billing Fraud
Once you have your Q code, you can now claim to gullible doctors that Medicare has “approved” your amniotic or umbilical cord tissue for whatever you snuck into the Q code description. Is that true? Of course not! You would also need to get a different part of Medicare to approve your medical use using a Local Coverage Determination. To do that, you would need to spend tens of millions on clinical trials proving that your use is safe and effective and 5-10 years pushing that rock uphill. However, this little short cut only cost 10-20K in consultant fees and takes a few months!
How the Scam Works
You can now begin submitting random bills to Medicare to find out which combination of ICD-10 diagnosis codes and CPT procedure codes gets the Medicare jackpot to spit out money (like scamming a broken Vegas slot machine). If you don’t get paid, then you appeal to Medicare using your Q code, trying to convince some low-level, didn’t graduate high-school, clerk that since your Q code says that your product can be used for knee arthritis, that they should pay the claim to treat knee arthritis. Is any of this legal? Of course not!
Why Would a Doctor Risk Federal Prison?
Why would a doctor take the risk of federal prison by billing this way? Given that these tissues are reimbursed at very high rates when used in ways Medicare allows (like wound healing), a doctor is reimbursed about $4,000 per 2 mls injected. The shady birth tissue vendor then signs a “Rebate Agreement” with the doctor so that while Medicare pays the doctor $4,000, the doctor only pays $3,200 for the tissue, so the doctor pockets $800 per 2 mls injected.
CMS Finds Out About the Scam and Begins Quoting FDA
You can easily find the documents that describe these CMS Q code approvals. I’ve reviewed a number over the past 6 months and was always surprised at no matter what crazy things the manufacturers placed in their descriptions of product use (like this stuff can treat knee arthritis without any data to support that statement), CMS seemed to rubber-stamp the request and grant a Q code. However, the CMS Q code document for the first quarter of 2020 was decidedly different. For the first time, we begin to see a significant number of denials.
Who got denied?
- Predictive Biotech-Corecyte-“After review of FDA’s guidance, it does not appear to CMS that the non-topical uses such as injection for cartilage repair that are also the subject of this application are appropriate for regulation solely under section 361 of the Public Health Service Act.”
- Predictive Biotech-Polycyte-also denied due to claims of “cartilage repair”.
- Predictive Biotech-Amniocyte
- Extremity Care LLL and careFLO
- Orangogenesis-ReNu-(applied for a J code and not a Q code)-Denied for knee OA despite a randomized controlled trial showing efficacy. CMS believed Renu was an unapproved drug product that wasn’t being legally marketed.
- Applied Biologics-Flograft-This company tried to get approval for: “FloGraft include those with acute and chronic wounds and soft tissue injury, muscle and meniscus tears, ligament and tendon sprains, degenerative tissue disorders and inflammatory conditions.”
However, CMS wasn’t consistent here. Despite these denials, Regenative Labs was able to get CoreText and ProText through with statements like, “They are typically used for muscle and cartilage tears and help to repair damaged tissue. The products are used for wounds and tissue defects and is applied directly to the defect using a syringe.”
So while CMS is catching on to the Q code scam, not all reviewers have been read in on which end is up.
The upshot? Is this the beginning of the end for the Q code scam? Certainly, based on one of the latest meetings, it looks like CMS is using the fact that FDA hasn’t approved these tissues for these uses to deny the Q code application. To me, that means at some level, someone in CMS knows that there’s a problem here.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
