Investing in Regenerative Medicine?
This week I was asked to speak to Wall Street analysts on many different companies in the regenerative medicine space. While fielding their questions, a number of themes kept emerging, so I thought I would write about those. Let’s dig in.
Regenerative Medicine Reality vs. Hype
Irrational hype has driven regenerative medicine investing from the start. So what’s real and what’s fiction? Which parts of the exuberance are real and which parts should be ignored? While I’ll focus on orthopedic offerings, much of what I’ll talk about in this blog applies to all clinical applications of stem cells.
I can break a bad investment in a regenerative medicine company into the following categories:
- Animal models
- Phase 1 garbage
- Making the blind see and the crippled walk
- Lipstick on a pig
- The HLA mismatch and the responder group
- Autologous competitors
Animal Models
The single biggest thing that needs to be ignored in regen med investing is what I call the animal model game. For example, cartilage repair and disc regeneration is very easy in rabbits and very hard in actual human patients. Meaning countless companies have tried products in rabbits or rats and have shown convincing evidence that holes in knee cartilage have been repaired and spinal discs regenerated, but at the end of the day, none could replicate those results in actual patients. Hence, the first rule in regenerative medicine investing is to ignore animal models. There simply isn’t enough cross over between rats or rabbits and human results for any impressive animal model results to convince me to put a dime into any company.
Phase 1 Garbage
Next up on stuff to ignore are phase 1 trial results. This is when the company is required by FDA to look at basic safety and oftentimes the results of these small trials are reported in press releases. The problem here? The results of treatment in a dozen patients is given monumental weight and then the company can’t reliably replicate any of these results when more patients are added in phase 2 and phase 3. So again, ignore phase 1 clinical trial data.
Making the Blind See and the Crippled Walk
One of the main early focuses in orthopedic stem cell therapy is often MRI results. This is called a “structural endpoint” in clinical trials slang. However, all too often, companies begin with these structural endpoints based on their animal and phase 1 trial data and then they’re dropped by phase 2.
Yesterday in my call to Wall Street I used Mesoblast as an example. Their initial concept for their stem cell product to treat degenerative disc disease was that it would regrow a new disc on MRI. However, after the Mesoblast phase II trial results were reported, this data was curiously deemphasized. Why? Because while it happened in rabbits, it didn’t happen in humans.
For example, way back in 2015 when that phase II trial was conducted, the company said:
“At 12 months, MPC-treated patients demonstrated a significant reduction in radiographically-determined translational movement of the disc, suggesting a treatment effect on disc degeneration, anatomy, and improved disc stability. The 18M MPC group had a mean translational movement of only 1.3%, the 6M MPC group 2%, the HA group 2.5%, and the saline group 3.5% (p=0.021 between groups). When adjusting translation per degree of rotation (TPDR), a similar treatment effect on reduced translational movement was seen in both the 6M and 18M MPC groups.”
Huh? What you’re being told here is that the company ditched looking for serious changes in how the low back discs held onto water and instead focused on an obtuse computerized analysis of instability not used clinically by 99% of physicians. Meaning they observed that the vertebral segment got a bit more stable after stem cells. Why focus on this? Because their major structural endpoint was widely missed, meaning they didn’t see discs regenerating due to the stem cell injection.
Hence, as I told the Wall Street folks yesterday, to justify the high price point of a product like Mesoblast, we need to see major results, not just pain relief. Those major results had better be changes on MRI. Meaning unless you’re making the blind see or the crippled walk, your expensive cell therapy product won’t be competitive.
Lipstick on a Pig
I also told the investors yesterday to be very careful about what is reported in the press releases on clinical trial results and to hire independent scientists to take them apart. Here we’ll look at Mesoblast and ViaDisc.
The companies that publish clinical trial results for stem cell disc regeneration products often try to put lipstick on a pig. Or as Mark Twain once said, there are lies, damn lies, and statistics. Let’s dive in.
The 2015 Mesoblast trial is worth revisiting here. The amount of pain using a 1-10 scale before the Mesoblast disc injection for all groups tested was about 7/10. The after treatment score for the high dose stem cell group was a 3/10. That’s pretty good as the pain dropped by 4 points or more than 50%! Impressive? Not really! The low dose stem cell injection group’s after procedure score was also about a 3/10. However, here’s where the wheels come off of the Mesoblast bus, the saline placebo injection had a pain score (patients injected with so stem cells) of about a 4/10! That means that all the high dose stem cell injection group could demonstrate was a lousy one point pain improvement over the placebo injection into the disc. Ouch!
Now let’s look at ViaDisc. This is an acellular extracellular matrix injected into the disc with the same goals as the Mesoblast DDD product. They ended a study last year with 220 patients and all I needed to see was this crazy graph to know that the lipstick was applied heavily on this data pig:
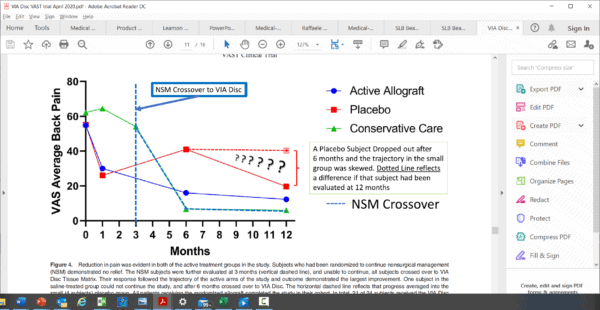
This is from a peer-reviewed publication and the authors of this paper have actually inserted question marks on the main graph describing the results. What the graph shows is that the placebo group line (red squares) had pretty similar results to the ViaDisc injection (blue circle line). Meaning both lines show the disability score going down. Then the authors inserted a dashed line postulating where the placebo group might have been if they didn’t lose a subject from the placebo group, which generated the question marks. How could losing a single subject make that much of a difference? The trial had a high ratio of patients getting the active therapy versus far fewer getting the placebo. However, the concept that the reviewers for this journal actually let this graph past peer review is absurd. Meaning that this is like saying, if our kicker had just not shanked it, the ball would have sailed through the uprights and we would have won the game.
The HLA Mismatch and the Responder Group
Allogeneic stem cells were supposed to be immune privileged, meaning that they were supposed to be incapable of provoking an immune response in the host despite being foreign tissue. However, recent stem cell research through Texas A&M has shown that this is only half true. Let’s dig in.
Getting back to Mesoblast above, several years ago I was lecturing at a major medical conference. On the podium in the same session about degenerative disc disease was one of the study investigators for the Mesoblast disc trial. What he pointed out was that their results were much better and separated from placebo more convincingly if you separated out a group of patients who were responders. This was about 40% of the total group. And indeed the data looked better that way.
The same thing happened with the Mesoblast knee arthritis study. About 1 in 5 patients had MRI evidence of improved meniscus size after receiving the stem cell injection compared to placebo controls. So again, here we have the concept of a responder group that actually met the structural endpoint that the rest of the group missed (better MRI findings after treatment).
Why is this happening? I would posit that this is because some of these allogeneic cells are being taken out by the host’s immune system. Let’s review.
What the Texas A&M data shows is that when you’re using someone else’s stem cells, they need to be HLA matched just like an umbilical cord stem cell treatment. Meaning the closer the match between the surface markers on the stem cells being injected and those on the host cells, the more likely it is that your stem cell treatment will work. If there is a bigger mismatch, you won’t get an immune rejection response, but your cells will be taken out by the host’s immune system. Hence, if you have a better matched donor, you get a 40% responder group. If you have a poorly matched donor, you get a 20% responder group. Hence, the fact that there are responder groups in many cell therapy clinical trials isn’t a good thing. In fact, it delineates a major flaw in the business plan of the cell therapy product, as these off the shelf stem cells from donors were supposed to be undetected by the patient’s immune system and that likely only half true.
Autologous Competitors
One of the recurring themes that kept resurfacing yesterday was the idea of cheap autologous therapies being competition for many of these expensive cell therapy products. A great example was Pluristem, which has a placental derived stem cell product that its pursuing for muscle/tendon injury after hip replacement. In fact, most of Pluristem’s published data is in the area of tendon injury. However, Pluristem has a huge PRP problem. Let me explain.
PRP is platelet rich plasma and it’s a comparatively inexpensive product compared to a high priced manufactured stem cell therapy drug. PRP has been shown to be effective in treating tendon injury in about a dozen randomized controlled trials. Hence, will an expensive cell therapy drug that requires a -80 C freezer to store be able to compete with a cheaper product that has already been shown to be effective?
As another example, take the two disc products discussed above. Once they either miss their structural endpoint like the Mesoblast product or can’t beat placebo like ViaDisc and resort to dashed lines on graphs, they have a serious problem. We already have a randomized controlled trial showing that PRP injection helps disc pain. Again, how does the expensive cell therapy product compete? It doesn’t.
Hence, any investor working in this space had better keep abreast of the evolving research for cheap autologous products like PRP. Not doing so is product suicide. Meaning if there are cheaper products that already have RCTs showing efficacy for the same clinical indication as your expensive cell therapy product, your product will never get widely used.
The upshot? I’m hoping that regenerative medicine investors can follow these simple analysis rules to make great investments. This field has tremendous potential and if you stay clear of products that fall into the above outlined traps, you’ll likely do well!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
