Magic Tricks, Electron Microscopy, and Umbilical Cord Tissue
Sleight of hand is a staple in magic tricks. Basically, you get the audience to look over here at an illusion, while the reality behind that illusion happens over there. While this works well on stage, it can also work in research and publications. After all, who really reads and dissects what research papers conclude and whether they can support that conclusion? This morning we’ll go over a paper that attempts to reclassify the regulatory nature of umbilical cord tissue using an electron microscopy magic trick. Let’s dig in.
Umbilical cord tissue vendors have a serious problem. As I’ll review below, the tissue they thought they could sell with a simple FDA online registration really requires full FDA approval with clinical trials. That has the effect of decreasing sales as physicians begin to get the memo that using these tissues to treat orthopedic problems could get them in trouble with the agency. Hence, they desperately need some help. In this case, that help comes in the form of a research paper that claims that the tissue matrix in umbilical cord Wharton’s Jelly products is almost identical to that contained in cartilage and muscle. Let’s dive into this fascinating story.
Umbilical Cord “Stem Cells”: Magic Tricks and Reality
For years the umbilical cord tissue sales industry got away with a major illusion. In fact, it’s still happening as we see patients deceived every day by providers using this sleight of hand. That trick was the assertion that umbilical cord tissue sold in a vial here in the US contained live, functional, and plentiful mesenchymal stem cells (MSCs). Then the reality set in, as multiple authors published research showing that for birth tissues this was fiction (1-3). In fact, our publication on umbilical cord products and stem cells that was published in the top orthopedic journal in the world laid bare this fiction (4). In the end, these products contained no live and functional MSCs at all.
Despite this reality, sales reps are still selling this stuff as MSC-rich. I know this because I have seen the sales emails and still see examples of medical providers who didn’t get the memo telling this falsehood to patients. Hence, I often tell patients that the mark of a provider who knows enough about this field to be dangerous is one who is still telling patients that umbilical cord products have lots of stem cells.
Medicare Clawback 101
The next big magic trick was the idea that amniotic and umbilical cord products could be reimbursed by medicare when used to treat knee arthritis. That illusion began when several vendors applied to get Q-codes for product reimbursement. These were issued with all sorts of creative descriptions for the use of these products which clearly violated FDA regulations. Despite these codes being issued, medicare made clear that it didn’t consider these products reimbursable when used to treat things like knee, hip, shoulder, or back pain. In fact, in talking with multiple orthopedic sales reps, Medicare has been retrospectively denying these claims one by one, causing expensive headaches for many practices who believed the umbilical cord vendors and their sales reps.
Regulatory Reality
While IMHO there is a clear fraud and abuse issue in the umbilical cord industry with misrepresentation of what these products contain and then bigger problems with the idea that medicare will reimburse a provider to use these to treat arthritis or pain, there’s an even more fatal issue. For years the FDA has sent Warning and other letters to umbilical cord vendors stating that these products when used to treat incurable diseases or orthopedic problems were unapproved drug products:
One of the disconnects between what the umbilical cord vendors want (a quickie 361 tissue registration) and what the FDA wants (a long and complex drug approval process) is “homologous use”. That means that in order for an umbilical cord product like Wharton’s Jelly to be a tissue and forego clinical trials, it needs to serve the same function in the patient as it served in the donor.
What is “homologous use for a WJ product wasn’t all that clear. However, the FDA recently clarified its homologous use criterion for these products to “serving as a conduit”, which is how the umbilical cord functions in a fetus. Hence, this eliminates all orthopedic use for these products and forces them all to go through an expensive and arduous regulatory approval process involving years of clinical trials. Most of the vendors got that final memo and either pulled their tissue products off the market or began those FDA approval trials. However, some are still out there selling their umbilical cord tissue as a 361 tissue without FDA 351 drug approval.
Using an Electron Microscope as a Magic Trick
Remember that a great magic trick uses distraction, but once you learn how it’s done, it seems obvious. If you’re an umbilical cord vendor trying to sell your product as a 361 tissue, you desperately need a trick or two up your sleeve right now. Hence, today we’ll review a paper published by employees/contractors at an umbilical cord tissue vendor that tries hard to use a microscope to change the regulatory classification of that product from a biologic drug back to a registered tissue. You might reasonably ask yourself, as I and many others did, how any microscope could accomplish that feat. Let’s dig into how the magicians involved accomplish that illusion.
Electron microscopy (EM) is an old technique that allows scientists to look at the structure of microscopic things. Recently, a research paper using EM on umbilical cord products was published and was touted as “one of the most significant papers to date in this space” by a sales rep for an umbilical cord product vendor called Regenative Labs:
So what was this EM research about? Let’s unpack what this paper states and some background. First, it’s interesting that the article was received by this small journal on 8/5/22 and accepted on 8/14/22. That’s so fast that I can’t believe that there was any significant blinded peer-review, which is a process that often takes months as copies of the paper are sent to scientists and physicians with expertise in the area and sent back and forth several times. In addition, it should be noted that several of the authors are non-scientists that work for Regenative Labs.
This paper focuses on two key regulatory areas: minimal manipulation and homologous use.
Minimal Manipulation
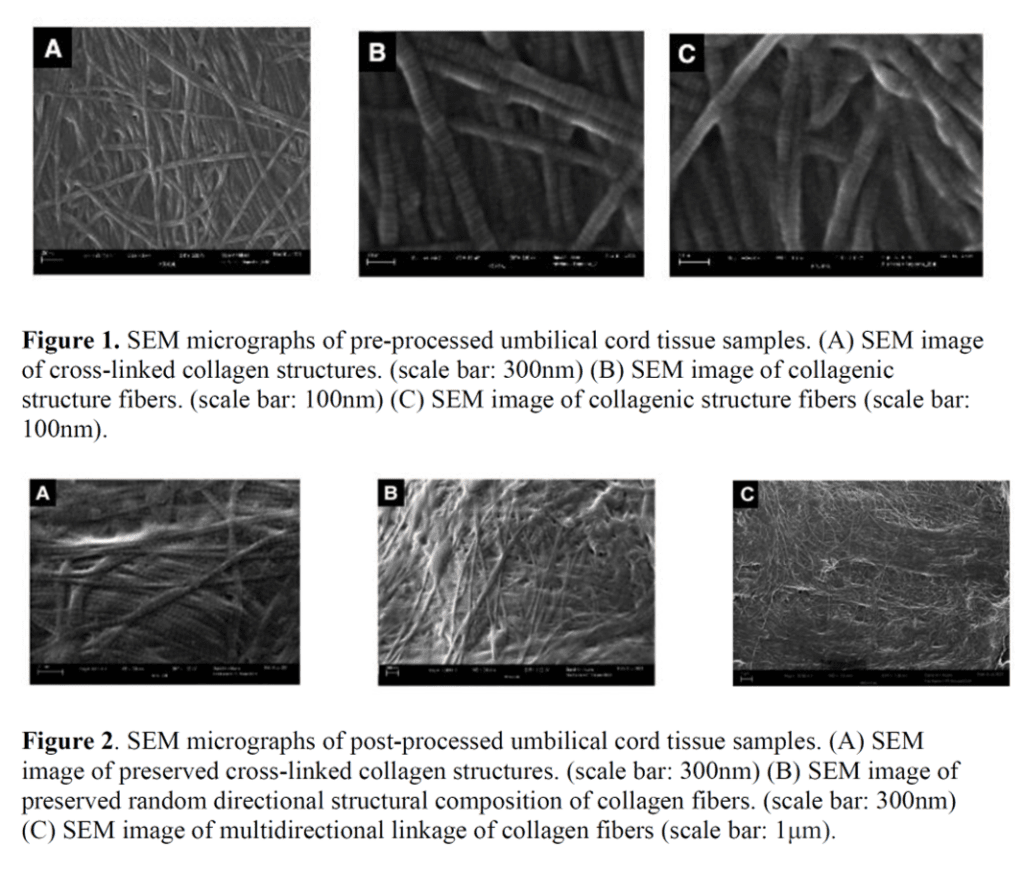
The article concludes that since WJ contains a fibrous architecture that can be seen on EM and that kind of looks similar at a microscopic level once you chop up the WJ into tiny pieces, then no significant changes occur as the WJ is processed into a commercial product. Why would this information be at all scientifically relevant? Because one of the FDA’s arguments has been that the act of micronizing WJ to allow it to be injected alters the relevant structural characteristics of the tissue. By doing that, it crosses a “minimal manipulation” line and becomes an unapproved drug product (see this letter). The language the FDA uses about WJ processing in these letters is:
“… your processing alters the original relevant characteristics of the umbilical cord and/or amniotic membrane related to their utility for reconstruction, repair, or replacement.”
So how does showing that the small bits still look the same under a microscope change that regulatory argument? It doesn’t. The language says that the umbilical vendors are changing the tissue’s ability to reconstruct, repair, or replace. No testing was performed on the ability of micronized WJ to repair anything. Nor does micronized WJ have the same biomechanical properties as non-micronized whole WJ tissue. In fact, that’s why it’s micronized, so it will fit through a needle, while unprocessed WJ is difficult to inject. Finally, the last bit on replacing tissue is dealt with in the next part of the study.
Homologous Use
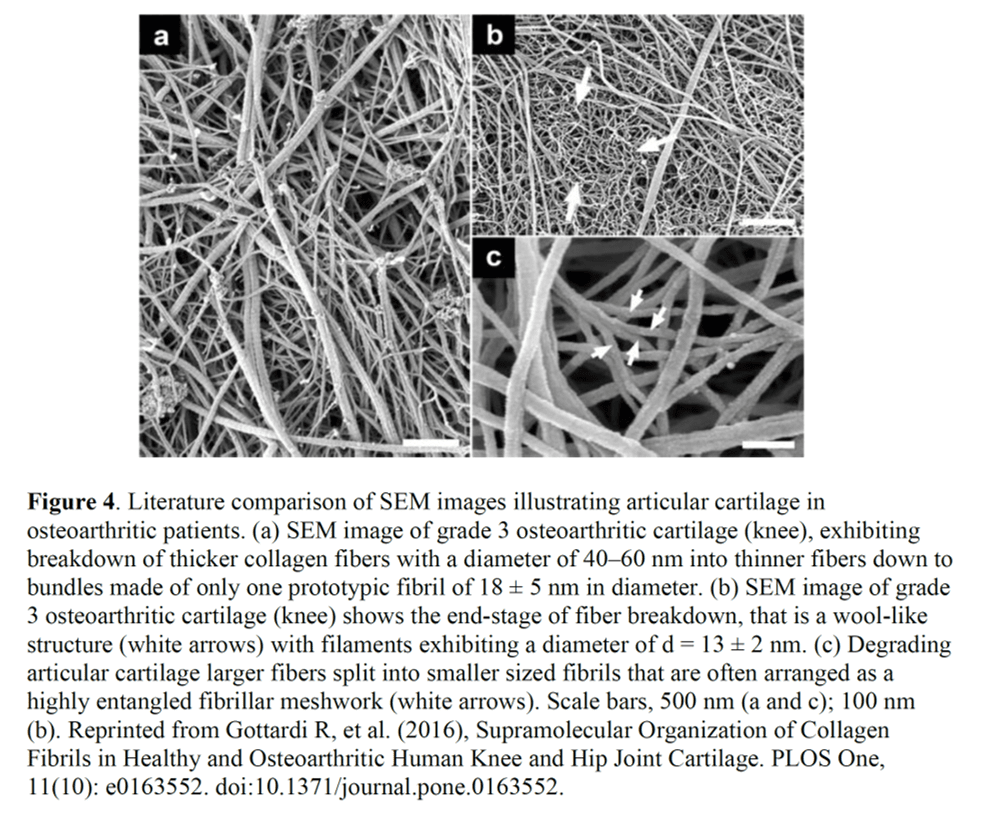
The next conclusion is that the WJ product under a microscope sort of looks similar to the mesh of collagen that can be seen when you digest away cartilage cells in a joint (or a muscle). Why would this be important? The authors conclude that this supports “homologous use”. Why use this term in a scientific paper? Because again, the goal here is to support that it’s OK to sell WJ under a 361 tissue registration without having to go through drug trials.
Will the FDA be convinced by any of this? Not in my opinion. This is now doubly true that they have redefined homologous use for umbilical cord products as “serving as a conduit”. In addition, just looking at the structure under a microscope would have little to do with passing the test that these FDA regulations are based on. For example, CFR 1271.1 states:
“…homologous use means the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor. “
The same basic function here would require that what makes up these WJ collagen fibers that were imaged on EM is the same or functions the same as the fibers in cartilage. Is that the case? Nope. For example, WJ contains type 1,3, and 5 collagen, while cartilage contains 95% type 2 collagen (5,6). Meaning that while the collagen fibrils may look similar on an electron micrograph, they are clearly NOT the same. That also means they function very differently. For example collagen 1 has very different mechanical properties than collagen 2, with type 1 being stiffer than type II (30% higher elastic modulus) (7).
In summary, collagen fibrils that kind of look similar on EM are NOT the same as far as composition and hence function, so they don’t meet the homologous use criteria in that they don’t serve “…the same basic function or functions in the recipient as in the donor”.
What’s Next?
If you read the Warning Letter tea leaves, this battle between the few remaining umbilical cord vendors and the FDA has seen new tactics being used by the agency. For example, note that most of the new Warning Letters involve a full inspection of the processing site and these facilities are being inspected like a Pfizer drug factory. Every single inspection has found serious deficiencies because these labs are operated at a less stringent GLP or GTP standard and not the full GMP required for drug production facilities. Hence, I suspect we will see more of these failed inspections.
In addition, there’s a whole new division involved here within CBER (Division 2). Meaning that the agency has committed funds and more employees to this effort.
Why is the FDA adding inspections? Because per their handbook, that’s what’s required to get to the next step in litigation. What’s that? Legal injunctions to shut down manufacturing.
The upshot? I suspect this new EM paper magic trick will convince nobody at FDA that a WJ product is homologous use for knee arthritis or other musculoskeletal problems. Nor does the paper add anything to our knowledge of how these products could help patients with orthopedic issues once they eventually get FDA drug approval.
____________________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. Am J Sports Med. 2019 Apr;47(5):1230-1235. doi: 10.1177/0363546519829034. Epub 2019 Mar 7. PMID: 30844295.
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Aug 16:3635465211031275. doi: 10.1177/03635465211031275. Epub ahead of print. PMID: 34398643.
(5) Sobolewski K, Bańkowski E, Chyczewski L, Jaworski S. Collagen and glycosaminoglycans of Wharton’s jelly. Biol Neonate. 1997;71(1):11-21. doi: 10.1159/000244392. PMID: 8996653.
(6) Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009 Nov;1(6):461-8. doi: 10.1177/1941738109350438. PMID: 23015907; PMCID: PMC3445147.
(7) Luo ZP, Sun YL, Fujii T, An KN. Single molecule mechanical properties of type II collagen and hyaluronan measured by optical tweezers. Biorheology. 2004;41(3-4):247-54. PMID: 15299257.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
