Major Medicare CAC Meeting on Amniotic for Orthopedic Use
As I’ve been writing for some time now, Medicare has now caught up to the dangerous game being sold by sales reps that physicians can bill Medicare for amniotic products used to treat orthopedic indications. As concrete proof of that, we have a major Medicare CAC meeting coming up in a few weeks on this exact topic. Let’s dig in.
Amniotic Fluid, Medicare, and Orthopedic Indications
Amniotic fluid was first sold as a “stem cell” product until research by many labs (including ours) showed that was fiction. The latest amniotic scam is using the fluid for orthopedic indications and billing Medicare despite the fact that no Medicare guidelines exist that show that this is covered. Medicare is being billed for these indications because a few companies were granted what’s called an investigational “Q-code”. However, the payment of these claims is happening because the part of Medicare that grants codes isn’t communicating with the part that creates guidelines.
Medicare Fraud and Abuse Contractors
Last year, a group of concerned physicians, acting through the non-profit IOF, sent a letter to every Medicare administrator opposing the coverage of these products due to lack of any clinical evidence that they worked well for these indications. That got the attention of Medical Fraud and Abuse contractors who were already tracking a steep uptick in claims being submitted for orthopedic conditions. Investigations are ongong into several players who have been instructing doctors to bill this stuff to Medicare.
Late last year, FDA got in on this most recent chapter of the Stem Cell Wild West. They began exercising their veto power when new amniotic and umbilical cord tissue companies tried to apply for their Q-codes. In addition, based on close contacts with the agency, they are also hot on the trail of companies who are billing Medicare.
A New CAC Meeting
The Medicare world is rich with acronyms. The CAC, MAC, and LCD are important here. What are those? This is from a Florida announcement of the new CAC meeting on amniotic products used for orthopedic indications:
“The Medicare administrative contractors (MACs) host multi-jurisdictional contractor advisory committee (CAC) meetings for the purpose of obtaining advice from CAC members and subject matter experts (SMEs) regarding the strength of published evidence for a specific topic. Review of the quality of evidence provided at the multi-jurisdictional CAC is used in development of collaborative local coverage determinations (LCDs).”
So what’s happening here is that one of the Medicare administrators (MACs, there are many private companies) is holding a meeting for all regions to get feedback from physicians about using amniotic products for orthopedic indications. This will then guide the review of the evidence which may update the existing Medicare guideline (LCD). To sign up to view the meeting, click here.
Why Is This Meeting Happening?
IMHO, this meeting is another milestone at the beginning of the end of this Medicare scam. Due to new transparency laws, the Medicare administrators must hold a public meeting on producing updates to existing guidelines. Note that the wording is around clinical evidence:
“The CAC panel will be asked to discuss the clinical literature related to Amniotic Products Injections for Musculoskeletal Indications, Non-Wound and rate their confidence in a series of Key Questions. Discussions will occur between the CAC panelists and MAC Contractor Medical Directors. The public may attend; however, questions from the public will not be entertained.”
Given that there isn’t much clinical evidence that these products work for orthopedic indications, you can expect this meeting to note that, which will generate a multi-region update to the Medicare guidelines (LCDs) that make it 100% crystal clear that these products are not covered for orthopedic uses. This is how the process works:
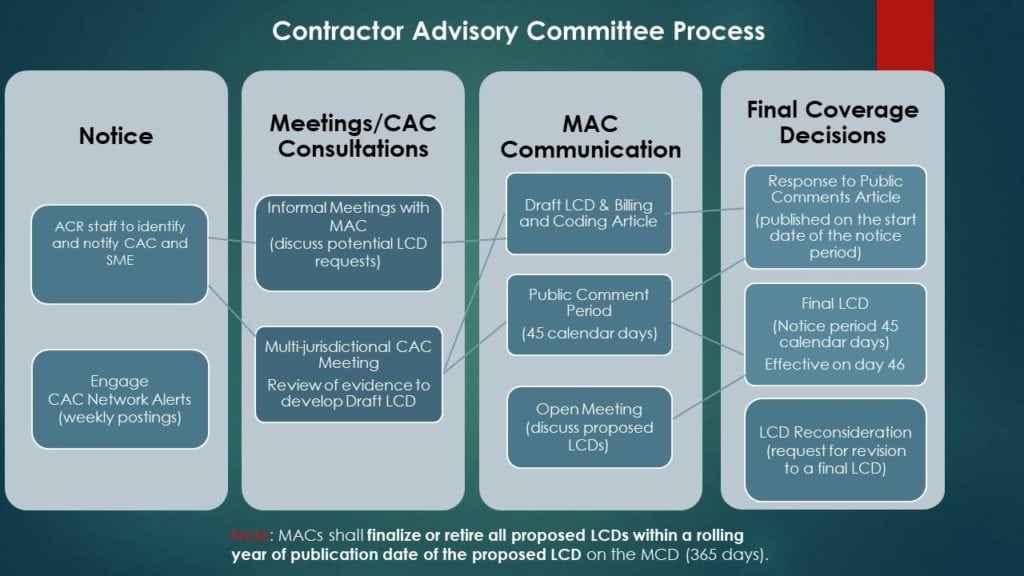
The upshot? IMHO, this new meeting is the beginning of the end of this scam. The word on the street is that this house of cards will soon implode.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
