Is Organicell’s Amniotic Fluid an Exosome, Nanoparticle, or Stem Cell Product?
I can’t go a single week without someone sending me something that highlights the crazy “stem cell” and exosome wild west. This week’s entry is a sales webinar put on by Organicell sales reps. After hours of research, I can’t figure out why the same amniotic fluid product has been marketed at different times as a stem cell, an exosome, and a nanoparticle product. Let’s dig in and learn a little about exosome products in the process.
What Are Exosomes?

Exosomes are used by all cells in your body, so this is not a phenomenon that’s unique to stem cells. It’s also critical to note that exosomes haven’t become popular because we have a slew of data that show that they can help your pain or arthritis like we do for PRP and bone marrow stem cells, but instead because of a failed regulatory theory. Let me explain.
When the FDA began to crack down on companies that were purporting to be selling amniotic and umbilical cord “stem cells” (which were really dead tissue), the companies playing that game switched strategies. They began to change their messaging from selling stem cell products to “exosomes”. They figured that if they were no longer selling “cells” the FDA would be fine with their products. Was this true? As you’ll see below, not so much.
The Exosome Concept
What’s the idea behind using exosomes for treatment? The concept is that instead of using the stem cells, the doctor uses the exosomes that the cells produce. Is this clinically validated that this will work? Nope. Nobody knows at this point whether this works in actual patients. In addition, as you’ll see below there are practical issues like:
- Making sure you only give the patient the right cell signals — this IS NOT trivial
- Making sure you actually have working exosomes in the mix — also NOT trivial
What Is Organicell?
This is a company in south Florida founded by two individuals from Ecuador who were involved in an autologous stem cell clinic called American Cellular & Anti-Aging Center in the city of Quito. One founder, Mari Maitrani claims to be an M.D./Ph.D., but her doctorate was from S.O.M.A. (Medical Society of Natural Therapies). I can find no university associated with that name, so it appears that this is not the same as a Ph.D. earned in an American university. I tried a Florida medical license look-up, Ms. Maitrani DOESN’T hold a Florida medical license.
The company’s sole focus seems to be on an amniotic fluid product. That product was called “Origanicell Flow” and is now known as “Zofin”. This is their FDA data:
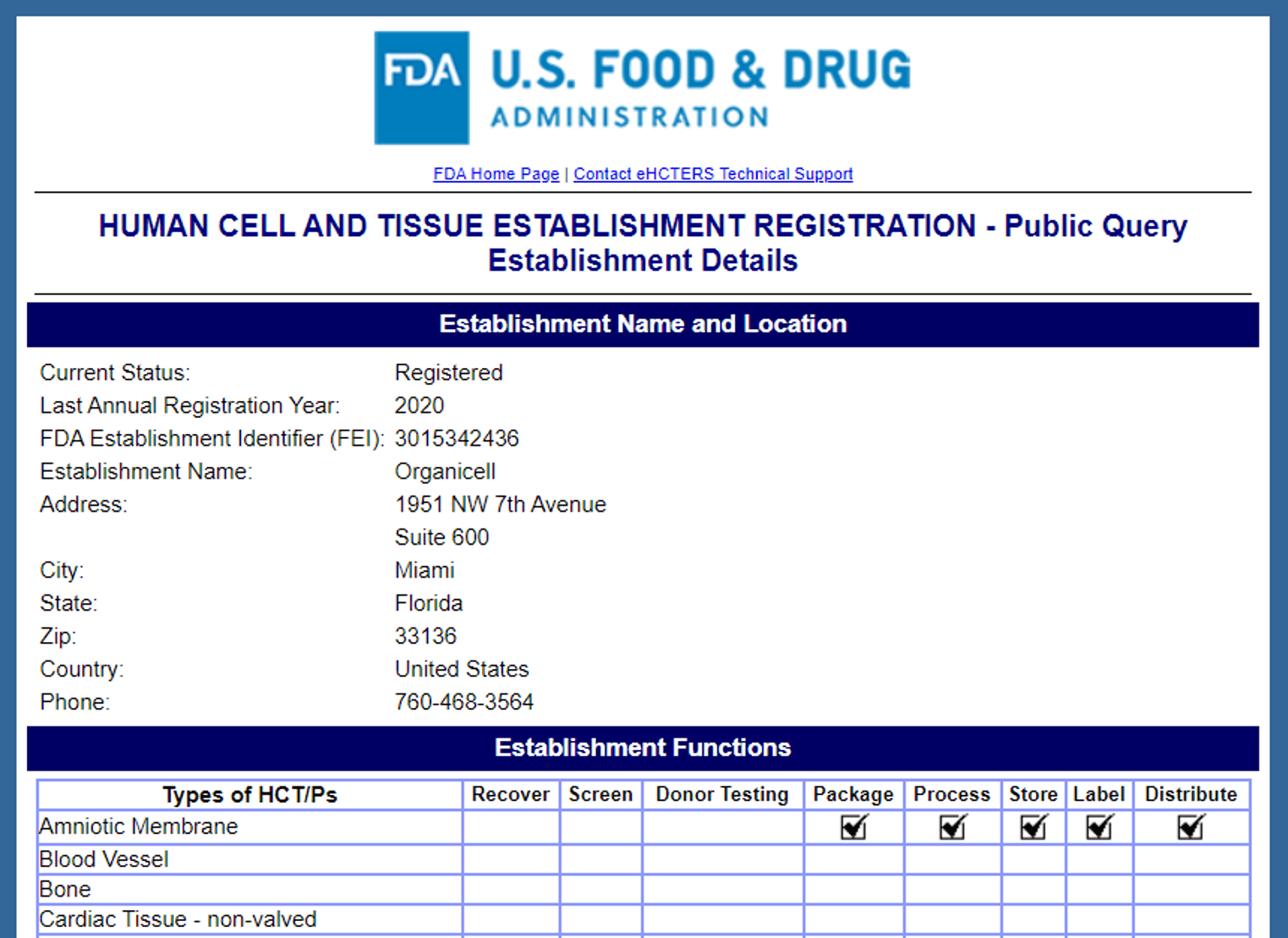
Note that this is a free 45-minute voluntary tissue registration without any FDA approval or clearance. They have listed that they can package, process, store, label, and distribute amniotic tissue. They do not recover, screen, or donor test these tissues (that’s apparently done by someone else).
Organicell was at the December 2019 A4M conference advertising with a booth that said “Exosomes – The Future of Stem Cell Therapy” See pic from the exhibitor space of that conference:

Who is in the picture? That appears to be the chief science officer Mari Maitrani (left) and their VP of Sales Tracy Yourke. That’s when their main product was known as “Organicell Flow”.
How Has the Company Previously Characterized Its Amniotic Fluid Product?
While the company claims that it sells a sophisticated nanoparticle product, in the past, the same product was sold as just plain old amniotic fluid. That offering also used to be called a “stem cell” product. Now all of that has morphed into a “nanoparticle” suspension. What gives?
In August of 2018, Oganicell Flow (now called Zofin) wasn’t described as a nanoparticle product. Here’s the description from the company’s website:
“Organicell™ Flow is a human tissue allograft derived from placental tissue, amniotic fluid and membrane.
Amniotic Fluid and Tissue are rich in components for tissue regeneration.
Including: Growth Factors, Cytokines, Collagen (all substrates: I, II, IV, V, VI). Fibronectin, Laminin, Hyaluronic Acid
Cellular components: Pluripotential MSC’s, microRNA, Associated Exosomes, Secretomes, Fibroblasts,
Keratinocytes, Epithelial cells”
Huh? While the idea that amniotic fluid may have exosomes is mentioned, it’s clearly not the focus of the marketing. However, there is a BOLD claim about the fluid containing mesenchymal stem cells (MSCs) that we now know was false based on testing from four independent labs on similar products (3-5).
By the following summer, (June 21, 2019) the marketing changed to:
So their amniotic fluid product that may have exosomes in 2018 has now morphed into an “exosome” product? Huh?
Let’s see what the marketing looks like today:

Hence, now the exact same amniotic fluid is being marketed as Nano-Particles Therapeutics”? So we went from just plain amniotic fluid containing stem cells (a false claim based on the published and presented literature) to an exosome product to a nanoparticle product? As I always say, you can’t make this stuff up!
Why Is a Physician Level Presentation Being Given by a Lay Salesperson?
Organicell recently gave a sales webinar on “Zofin” (Organicell Flow) that was sent to me. First, while I’m used to physicians or scientists giving presentations to doctors on products, I’m not used to a webinar for physicians being given by a salesperson (in this case Tracey Yourke who holds a 4-year business degree):
The 30,000-foot view from the webinar is that this is a nanoparticle product. However, the company goes back and forth between the terms nanoparticle, extracellular vesicles, and exosomes. Why the confusing language? IMHO, it’s because the FDA has called out those companies vending exosomes as selling illegal drug products:
In watching the whole presentation, there was ZERO CLINICAL DATA presented showing that the amniotic fluid being sold (Zofin) would help any orthopedic condition. In addition, all sorts of claims are made which IMHO are not permitted:
- The product can regenerate organs
- The product has exosomes
- That Zofin is “The COVID Solution”
In fact, that last claim is baked into the logo for Zofin:

First, let’s dig into whether this is an exosome product and then we’ll get into COVID-19.
Identifying Exosomes?
How can we identify exosomes? The problem is that this is NOT trivial. Here’s what’s usually done:
- Looking for very small particles. This can be done with a machine called a “NanoSight”. This is not able to ID exosomes by itself, as everything from cell debris to air bubbles as well as exosomes will show up the same. Organicell says it offers a NanoSight report with every batch, but again, as my Dad used to say, this and a Nickel will get you a cup of coffee.
- Markers on cell surfaces. This is done with flow cytometry, but again, the problem is that these markers aren’t specific for exosomes. This is what the company claims is in its product:
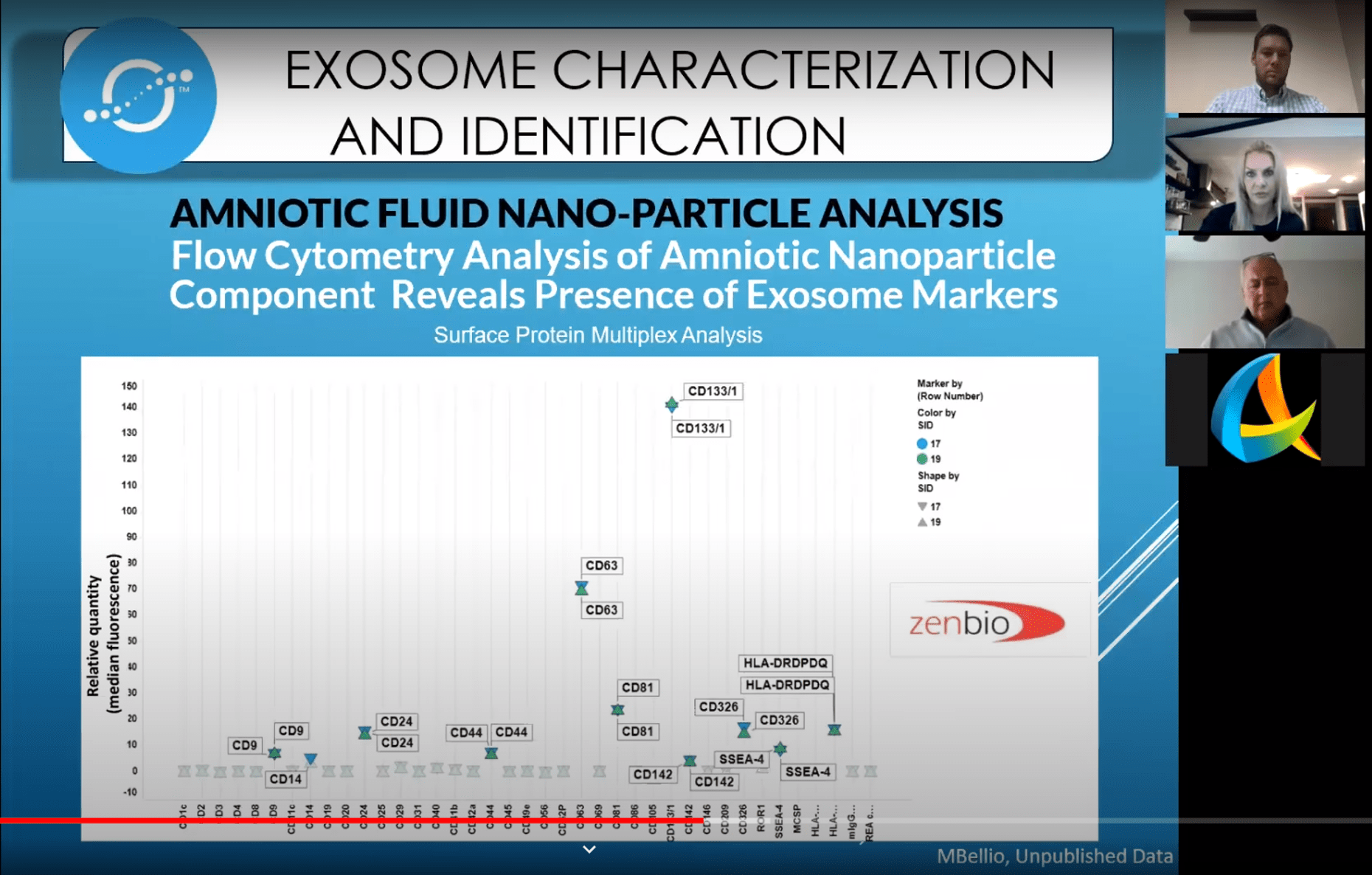
- CD133 is a cancer stem cell marker
- CD63 is associated with extracellular vesicles, but again, also associated with other things
Hence, looking for small particles and performing flow cytometry won’t get you convincing data that your product has exosomes that could help patients. In fact, you won’t know how many of each type of exosome is in the product nor will you know if any can still have an effect on the patient’s cells.
Proprietary Processing?
Organicell states that Zofin is made with “proprietary processing”. This amniotic fluid product is NOT an FDA approved and reviewed drug, but merely a registered tissue. Hence, what the company can deliver to physicians is extremely limited by FDA guidelines. For example, the company can only use simple filtration and centrifugation which SEVERELY LIMITS the processing. For example, it can’t decide to try to use sophisticated techniques to choose certain exosomes, as that would make this an unapproved drug product. Hence it’s focusing on the moving target of “naturally occurring exosomes”.
Heterogeneity
Our lab team has found HUGE differences in the growth factor levels in various amniotic fluid products and various lots of the same product. You can bet this also holds true for exosomes. Is there a way to make sure that each lot has consistent exosomes that can help specific problems? Not in 2020.
Problems with Exosome Products that Have Still Yet to be Solved
There are some serious issues with delivering an exosome or “nanoparticle” product in 2020, not the least of which are:
- Choosing which exosomes are pro-repair, which are noise, which could be harmful
- Targeting exosomes to specific tissues
- Naturally occurring exosomes have short half-lives
On #1, we have no way right now to choose the right exosomes. For #2, read this statement from a scientific paper on exosomes:
“For the development of exosome-based therapeutics, it is important to control the pharmacokinetics of exosomes, that is, the selective delivery of exosomes to target organs and cells. To control the pharmacokinetics of exosomes, the modification of the surface of exosomes with targeting proteins or peptides has been widely used.” (1)
Meaning that when exosomes are used in experiments, they are often modified in ways that these companies can’t accomplish and still stay within a tissue registration.
For #3, how long can you store exosomes? How about this statement from a scientific paper:
“Biological fluids (e.g., plasma) containing exosomes can be stored for up to 5 days at 4°C in a glass bottle (Théry et al., 2006).”(2)
In other words, exosomes don’t hang around very long even when refrigerated, let alone at room temperature. This makes common sense as orders barked from a cell would be specific to the microenvironment at the time and may not apply even hours later.
So we don’t know which exosomes to sell you, we don’t know how to modify those exosomes so they act only on specific tissues, and what manufacturers deliver is likely non-viable in that the half-life of exosomes is very short.
COVID-19
This year the FDA offered manufacturers a unique opportunity. They could get short-cut approvals to try their drug against COVID-19. Zofin was granted one of those approvals, which is great. However, this is an approval to perform a tiny phase 1/2 safety study, and DOESNT allow the company to issue a claim that Zofin is “The COVID Solution”. First, Zofin has not been approved by FDA as a drug to treat all COVID-19, just authorized for a small closely monitored study. Second, the language around drug marketing is highly regulated. Meaning there’s a reason that you don’t see your box of antibiotic pills say something like “The Srep Throat Solution” as no manufacturer could ever get that type of language approved.
Conclusions?
This product went from a magic amniotic fluid product containing stem cells in 2018 to an exosome product in 2019, and then once the FDA cracked done on the claims of “exosome” companies, it morphed to a “nanoparticle” product. As you can see, while nobody knows if Zofin does anything for pain or any other clinical condition, the company is busy aggressively marketing this “stem cell”/exosome/nanoparticle product. Is this permitted per FDA regulations? Not in my opinion. So is Organicell’s amniotic fluid a “Stem Cell”, “Exosome”, or “Nanoparticle” product? Your guess is as good as mine.
____________________________________________
(1) Morishita M, Takahashi Y, Nishikawa M, Takakura Y. Pharmacokinetics of Exosomes-An Important Factor for Elucidating the Biological Roles of Exosomes and for the Development of Exosome-Based Therapeutics. J Pharm Sci. 2017 Sep;106(9):2265-2269. doi: 10.1016/j.xphs.2017.02.030. Epub 2017 Mar 7. PMID: 28283433.
(2) Akuma Precious, Okagu Ogadimma D., Udenigwe Chibuike C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst., 16 April 2019 | https://doi.org/10.3389/fsufs.2019.00023
(3) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(4) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(5) Fitzsimmons ED, Bajaj T. Embryology, Amniotic Fluid. [Updated 2019 May 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541089/

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
