Our Latest Publications
Regenexx has always had an active research program with our goal being to try and get a few papers published every year. However, COVID slowed down the publication process for everyone. So we have a bunch of publications coming out all at once now. Let’s dig in.
Our Publication Team
Yesterday, during one of our research team meetings, we all discussed how much time each and every published paper takes. Dozens of hours of pulling, reviewing, and analyzing data. Dozens of more hours writing and editing and formatting to meet a specific journal’s requirements. Then many more hours spent going back and forth with reviewers. So by the time the average scientific paper gets published, there are 100+ to hundreds of hours of work sunk into it.
To get all of that done, we have a team that consists of:
- Chief Medical Officier – that’s me
- Chief Science Officier – Neven Steinmetz, PhD
- Clinical Research Director – Ehren Dodson, Ph.D.
- Biostatistician – Ian Stemper
- Jack of All Trades and MVP (Science Team member and Head of our Research Lab) – Dustin Berger, M.S.
- Our Clinical Fellow or Fellows
The New Publications
We have one new clinical research paper and one paper that I won’t claim ownership over at all, but our publication team was critical in getting out the door and published.
Bone Injections R Us
One of the things that separate the experts in this area from the pretenders is the ability to perform advanced procedures like injecting bone. For treating knee arthritis, this has been a big buzz among legitimate physicians performing Orthobiologic procedures. Does injecting the bone as well as the joint improve outcomes? If so, how should that be done?
There are two main techniques used by physicians who are trying to improve patient outcomes by injecting bone. One is a low-volume version where the provider tries to target a specific bone marrow lesion seen on imaging using x-ray guidance. The other would be injecting much more volume into the side that has the most pain, regardless of whether the patient had bone swelling in the bone.
We’ve had our data analysis done for some time now, but COVID put a damper on getting this published. So what did we find in knee arthritis patients when we tested the first method against just injecting the joint? (1) There was no difference. That’s the opposite of what Phillipe Hernigou found using the second high-volume method discussed above (2,3). So we did our part in testing these two approaches and are thankful that one of them worked to improve outcomes in this procedure.
Delphi Panel
The world of orthobiologoics desperately needs standards. This next paper is not a Regenexx paper but is one that I personally pulled together and then the Regenexx research team helped get out the door. For this, we pulled together an expert panel of academics and private practice physicians who all voted and tried to reach a consensus on various critical topics in the use of bone marrow concentrate in Orthopedics. This is called a Delphi panel and here’s some of what they all agreed to:
- The use of a treatment registry
- The need for candidacy grading each patient
- Using expanded informed consent
- Being scientifically accurate in advertising
- The use of an IRB when novel uses are tried
- Imaging guidance for injections
- The publication of results in the peer-reviewed literature
Finally, this last part is critical. Many bench scientists have made up a standard that all medical care with Orthobiologics must have high levels of evidence like many randomized controlled trials showing efficacy before they can be used in patients. However, our panel of experts who are actual doctors licensed to treat patients chose a different standard. They decided that the minimum requirement is case-series level data before routine use of bone marrow concentrate in Orthopedics.
More Regenexx Research
These two new papers as well as many more that we have published or helped facilitate can be found at this link.
The upshot? Research takes a long time and is expensive. However, for a new field, it’s critical that people invest that time and energy, which is what we have always tried to do at Regenexx. So enjoy these two new papers!
__________________________________
References:
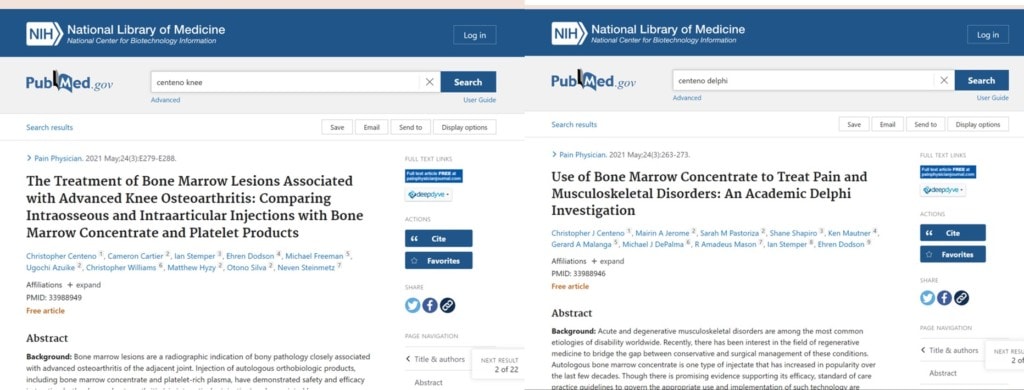
(1) Centeno C, Cartier C, Stemper I, Dodson E, Freeman M, Azuike U, Williams C, Hyzy M, Silva O, Steinmetz N. The Treatment of Bone Marrow Lesions Associated with Advanced Knee Osteoarthritis: Comparing Intraosseous and Intraarticular Injections with Bone Marrow Concentrate and Platelet Products. Pain Physician. 2021 May;24(3):E279-E288. PMID: 33988949.
(2) Hernigou P, Bouthors C, Bastard C, Flouzat Lachaniette CH, Rouard H, Dubory A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop. 2021 Feb;45(2):391-399. doi: 10.1007/s00264-020-04687-7. Epub 2020 Jul 2. PMID: 32617651.
(3) Hernigou P, Delambre J, Quiennec S, Poignard A. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int Orthop. 2021 Feb;45(2):365-373. doi: 10.1007/s00264-020-04571-4. Epub 2020 Apr 23. PMID: 32322943.
(4) Centeno CJ, Jerome MA, Pastoriza SM, Shapiro S, Mautner K, Malanga GA, DePalma MJ, Mason RA, Stemper I, Dodson E. Use of Bone Marrow Concentrate to Treat Pain and Musculoskeletal Disorders: An Academic Delphi Investigation. Pain Physician. 2021 May;24(3):263-273. PMID: 33988946.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
