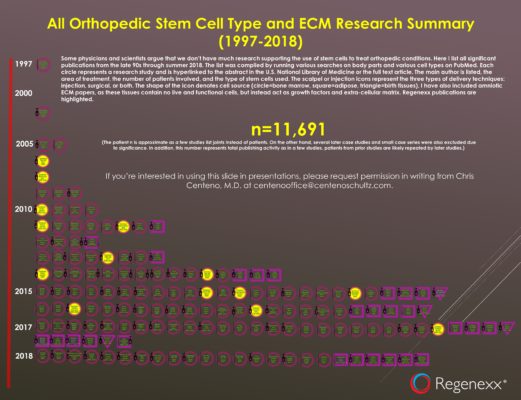
My All Stem Cell Type Orthopedic Stem Cell Research Infographic for 2018
(Click here for a larger view)
A few days ago, I published my 2018 version of the orthopedic bone marrow stem cell research infographic. Since last year, I’ve also published an annual all cell types version, which adds in the research being published on adipose (fat) and birth tissue stem cells. This year I also threw in ECM products (like amniotic fluid and membrane). So what did I find that’s new?
What Are the Trends?
First, just as last year, Asia is dominating the fat stem cell research field in orthopedics. This is a bit concerning as most of this research is occurring outside of the U.S. Not sure what that means for the idea that the U.S. is the medical research engine of the world.
In adipose research over the past 14 months, there have been studies mostly on culture-expanded adipose stem cells and a few on same-day stromal vascular fraction procedures. There have also been a few small publications where microfractured fat (Lipogems) was used. While this is not a stem cell procedure, it may have some very real clinical utility in arthritis and other areas. See my video below on Lipogems and stem cells:
There are a few publications on birth tissue-derived stem cells, but realize that these use isolated and culture-expanded cells harvested fresh from donor deliveries and not commercially available whole tissue products (which don’t contain live and functional stem cells). Again, these are mostly out of Asia. As for research on commercially available amniotic or cord blood products, there isn’t much.
The Birth Tissues Practice Versus Research Grand Canyon
The clinical use of birth tissues like amniotic fluid and membrane, Wharton’s jelly, and umbilical cord blood has exploded, largely due to the sales rep- and manufacturer-created fiction that these commercial 361-registered tissue products have live and functional stem cells. What’s fascinating is that all of this is being done with basically no or extremely limited research. For example, to date, here’s what I could find on studies that used these products (total patient n published to date):
- Achilles tendinitis and plantar fascia—n=44 (paper doesn’t say how many with each diagnosis treated)
- Knee osteoarthritis—n=6
- Spine—n=0
- Shoulder—n=0
- Hip—n=0
That’s it! Given that most of what’s being treated by nurses in chiro clinics is either knee or hip arthritis or spine by pain management clinics, all of that activity and tens of thousands of treatments a year are solely based on 6 knee arthritis patients, no hip research, and not a single spine-focused patient study. So if you’re using these tissues as stand-alone therapies, you are pushing the envelope here and risking medical board, AG, or FTC action based on making claims not supported by the peer-reviewed literature.
The State of Fat Is Where It’s At
The good news for those using fat grafts, microfragmented fat, and SVF is that there are now a few more small publications. The bad news is that most of the growth of the publications in this space over the last 14 months used culture-expanded adipose stem cells and not these simpler products. Again, we have a huge divide here between what’s being done out there and the research. Let’s take microfragmented fat (Lipogems). Here’s the total publishing activity:
- Knee arthritis—n=36 (injection) + n=30 (surgery) + n=17 (injection)
- Spine—n=0
- Shoulder—n=18
- Hip—n=0
- Foot/Ankle—n=0
Hence, if you’re injecting Lipogems for knee arthritis, you’re hanging your hat on 71 published patients, which isn’t even enough to determine safety. In addition, there isn’t a single publication in the spine or other areas like the hip.
While there are more publications and a larger n using SVF (digested fat which is spun down to isolate a fraction containing stem cells), it’s now illegal in the U.S.
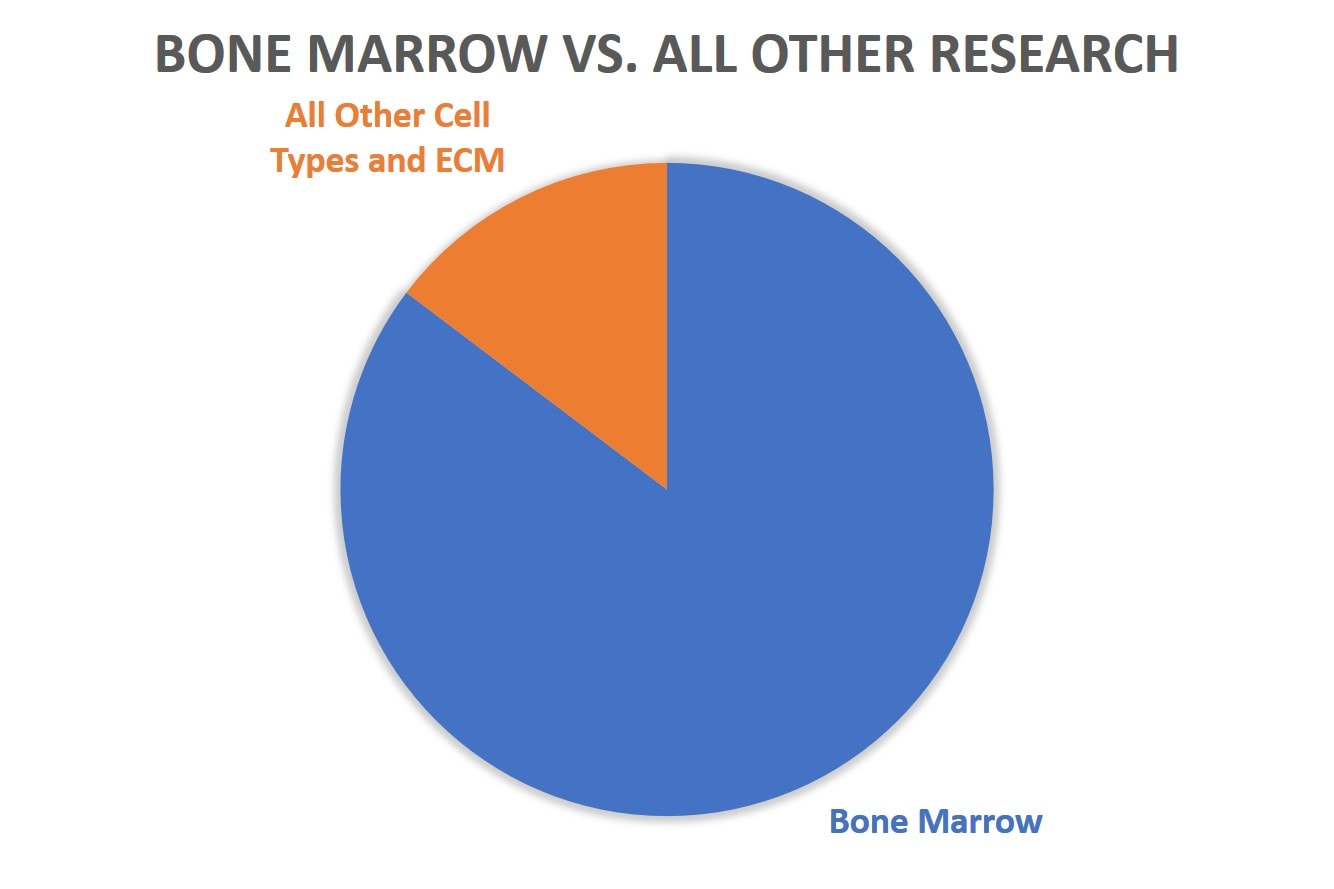
The Bone Marrow Research King Is Very Much Alive
While birth tissue 361 product research is almost nonexistent and Lipogems has some data, but not much, and SVF a little more, bone marrow as a tissue source for stem cells is still the big dog on the block. Here’s a comparison of the number of patients treated for bone marrow versus all other cell types:
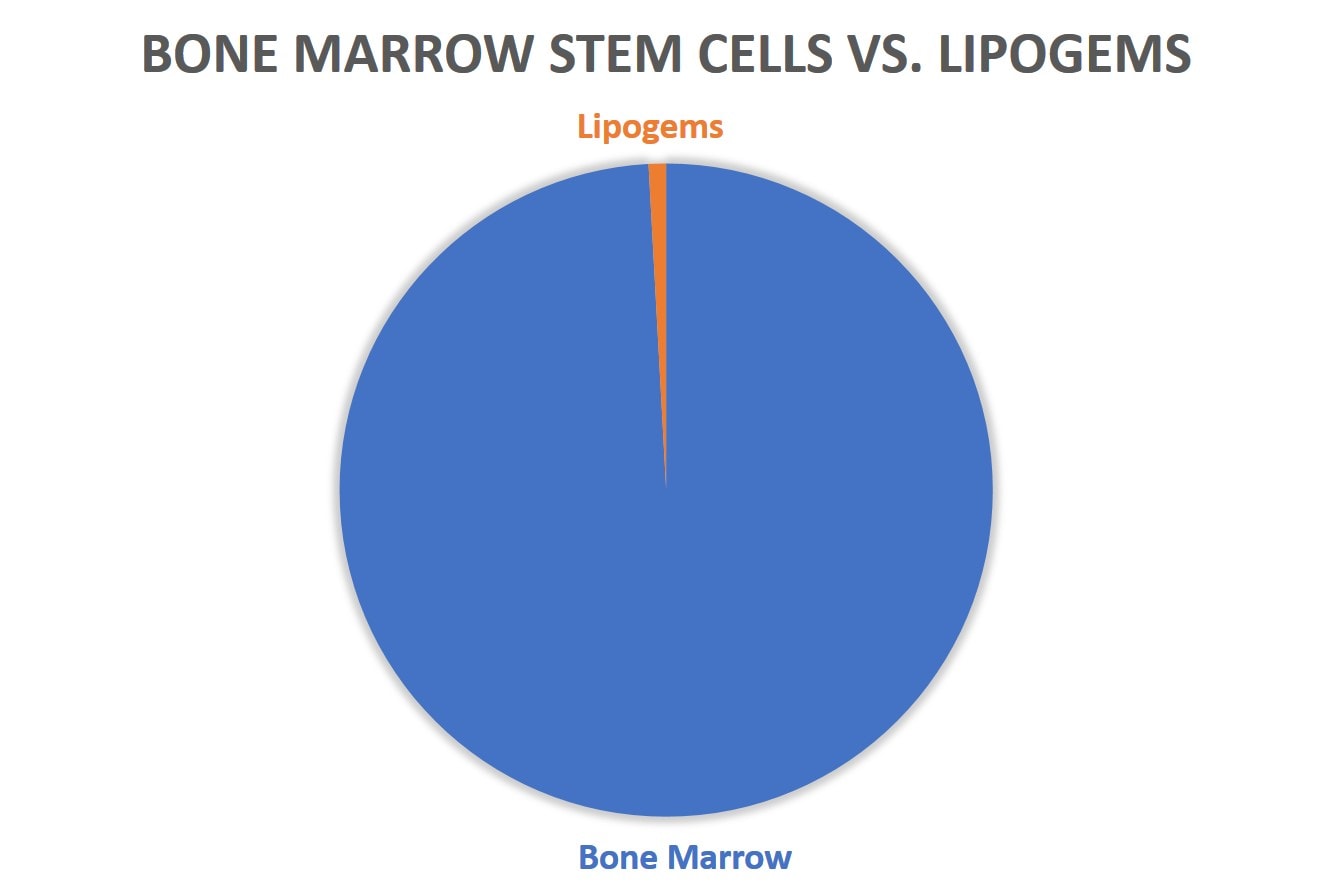
The disparity gets even bigger once we start comparing bone marrow stem cell research to specific treatments, like Lipogems:
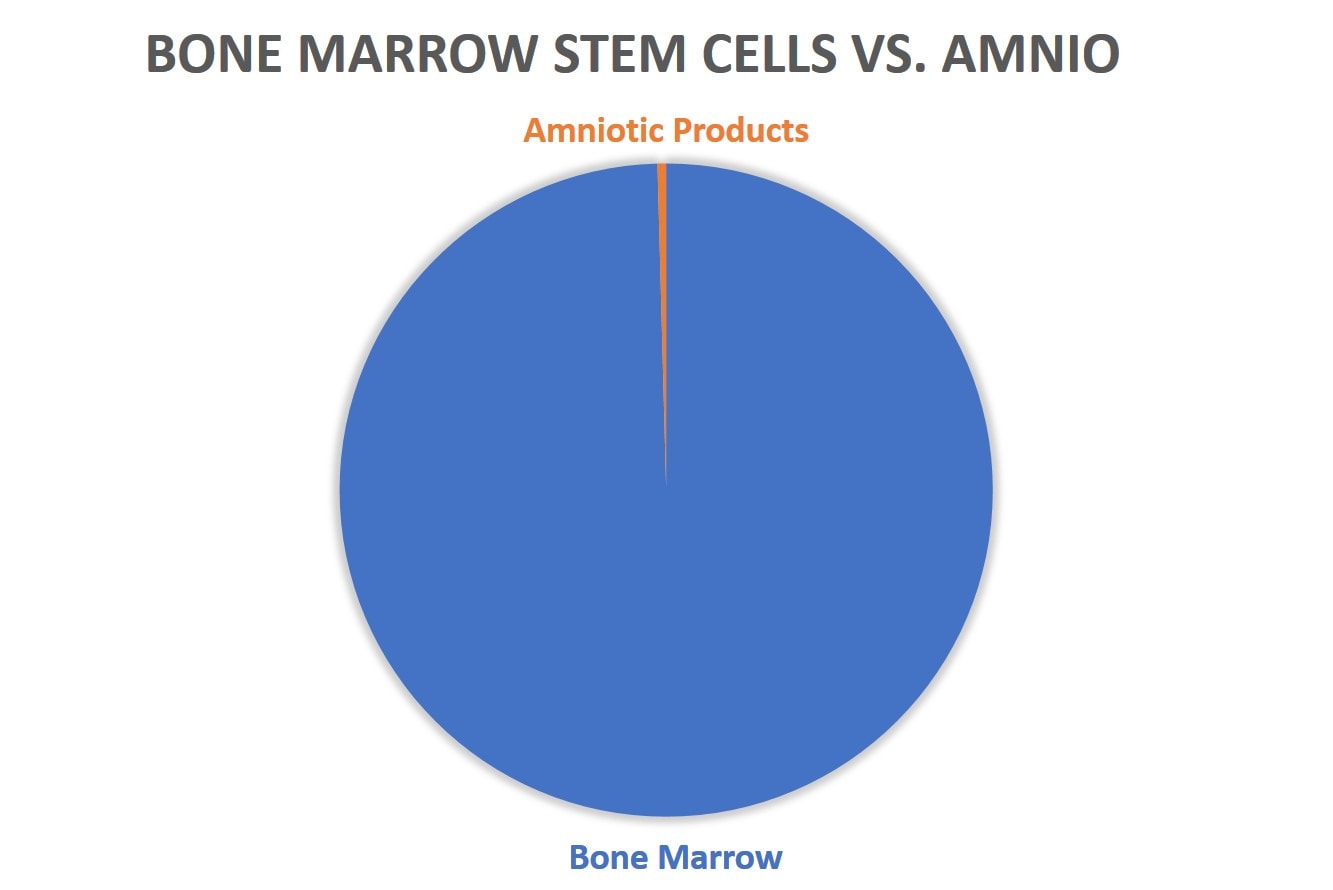
And even worse if we compare bone marrow research to all data published on commercially available birth tissues (here amniotic membrane and fluid):
The BMA Magic Needle Two-Step
Another disturbing trend this past 24 months has been devices like the Marrow Cellutions (aka RegenMaxx and now the Maxx-Cell). These purport to allow you to draw bone marrow and not have to isolate the stem cell fraction via centrifugation. Regrettably, our own research studies plus those of others have shown that these devices do not concentrate the stem cells and produce “stem cell poor” product compared to what can be done with the addition of a centrifuge (bone marrow concentrate). Despite this, we have seen a number of doctors adopt these devices. So how much research did I find on this new type of therapy (i.e., bone marrow aspirate created by these devices and used “as is”)?
- Knee arthritis—n=0
- Spine—n=0
- Shoulder—n=0
- Hip—n=0
- Foot/Ankle—n=0
Yep, there is exactly no research on patients treated with the product of these devices!
The Ethics of Offering Orthobiologic Treatments with Limited Research
When we first began offering stem cell treatment for orthopedic issues in 2007, it was after treating more than 50 patients for free as part of an IRB-approved study from 2005–2007 and carefully tracking all of their outcomes in a registry. We also took before and after MRIs on every patient as well as blood work. Hence, in late 2007, I felt comfortable enough with the results to begin charging patients. However, compare and contrast that to what’s happening now.
First, let’s take Lipogems. The orthopedic surgeon who takes a course and then begins hog-wild treating patients, given this limited data set, is on the ethical edge. Now, if that physician is carefully tracking outcomes (hat tip to Richard Striano and Gerry Malanga, who published one of the papers using Lipogems), then that’s a different story.
Now let’s look at amnio products. The chiro or pain management doctor who wants to hang his hat on exactly 50 published patient results, and try to defend that in court if a suit is filed, deserves to go down in flames. Again, if that provider is carefully tracking outcomes and complications and lets the patient know that there is VERY limited published data, that’s a different story.
Finally, the Marrow Cellutions crowd (or Maxx-Regen or Maxx-Cell) has ZERO published data to fall back on. None, zero, zippo, zilch. Your honor, I find for the plaintiffs…Again, a provider using these devices and capturing outcomes and complications who lets the patient know that there is NO published research is in the clear.
You get the drill, right? If you’re using bone marrow concentrate, the data is strong enough where you can begin to just treat patients. If you’re using anything else, you better be tracking outcomes and complications in a regimented patient registry or similar.
The upshot? This yearly dive into the research takes a huge amount of time. If you know of anything important I missed or find a bad link, e-mail me at [email protected]. In the meantime, while culture-expanded fat stem cell publications out of Asia and a few on Lipogems are making some headway, the lion share of the data is still with bone marrow-based stem cell therapies.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.