Umbilical Cord Stem Cell Therapy for Knees
You may have gone to, or are planning to go to, a local Seminar to get your knees treated with an umbilical cord stem cell therapy. Let’s dig into the sales pitch and see if it holds water. After all, these treatments can run thousands to tens of thousands of dollars. So is this the real deal or something else?
The Basic Concept Behind Stem Cell Therapy for Knees
The basic idea behind umbilical cord stem cell therapy for knees starts with animal research showing that mesenchymal stem cells (MSCs) can help knee arthritis. The first papers in humans that used MSCs to treat knee problems were actually published by myself way back in 2008, long before there was a single stem cell clinic operating in the United States (1,2). Our early research actually used a completely different type of stem cells, those derived from the patient’s bone marrow. Since then, we have published several more papers on treating knee arthritis using bone marrow concentrate (BMC), which is the same day version of the cultured cell procedure we originally pioneered (3-5).
Research Update: Umbilical Cord Stem Cell Therapy for Knees
While you may not know it, it’s easy to see what research doctors have or haven’t published on a specific topic. To do that here for umbilical cord stem cell therapy for knees, I went to https://www.ncbi.nlm.nih.gov/pubmed and typed in “Umbilical cord knee osteoarthritis”. While there are a few clinical studies involving patients, all studies involving umbilical cord stem cells used products that had isolated cells that were then grown in culture, so, not anything that’s legal in the United States (6,7). None of these studies used the type of umbilical cord tissue used by the doctors giving seminars. So let’s dig into that further.
What Type of Umbilical Cord Stem Cell Therapy for Knees is Being Offered in Seminars?
The type of umbilical cord stem cell therapy for knees being offered in seminars is an FDA 361 registered tissue graft that only has minimal processing. This is not an FDA approved product, but one that is simply registered on an FDA website. This lack of drug type oversight is what recently led to the serious contamination of umbilical cord products that caused serious illness in many states (8).
These tissue grafts are night and day different from the type of culture-expanded, umbilical cord stem cell therapy described above that was used in a handful of clinical trials. The biggest difference is that in the later the umbilical cord is fresh and then the cells are harvested, processed, and placed in culture to grow more. That’s not legal in the United States as this would be a new drug product requiring FDA approval through clinical trials. What is legal in the US is to just take the umbilical cord tissue, process it, and freeze it for sale. The problem with that type of product is that university and 3rd party studies have shown that there are no viable and functional stem cells when these products arrive and are thawed at a clinic (9-11). Hence, the small amount of research above in patients that used the cultured and isolated umbilical cord stem cells that were living can’t be compared to the dead umbilical cord cells being used in clinics.
There’s an old pirate saying, “Dead men tell no tales”. You can apply that here as well, as dead stem cells have no effects. Meaning a living stem cell is required for stem cells to function to orchestrate tissue repair.
Beware of the Research Bait and Switch
The most common thing I see when umbilical cord clinics list research, is that they list zero research on the dead cell product they’re actually using. What they do list is either research like the papers we have published on bone marrow stem cells or research on the cultured umbilical cord procedure I listed above. That’s obviously like comparing apples and oranges, but they hope you don’t know enough to catch the bait and switch.
How Do Umbilical Cord Stem Cell Products Compare to Middle-Aged and Elderly Bone Marrow on Stem Cells?
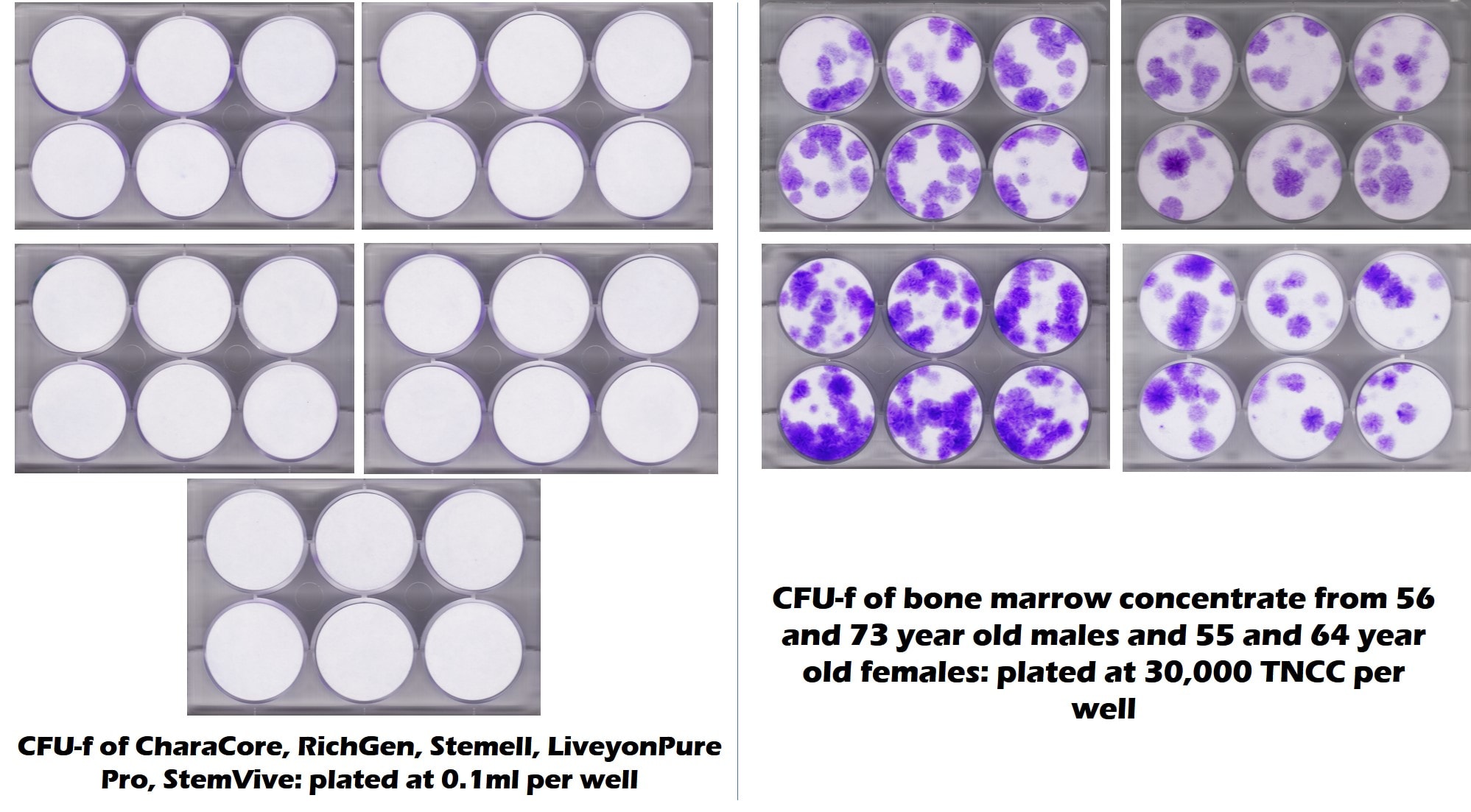
There’s nothing better than a picture to convey a message. The images below are of tests run by our lab and a university as a joint project to look at stem cell content of five common umbilical cord products used by clinics. The purple dots represent stem cell colonies and white is no stem cells. On the left, you see 6-well culture plates on the five common umbilical cord products and on the right you see bone marrow from late middle-aged and elderly patients. There are no living stem cells in any of the 5 umbilical cord products tested, but plenty in the elderly bone marrow. This is the opposite of what the seminars tell you!
Before and After Knee X-rays Shown in Seminars
One of the more popular things to show in seminars that tout umbilical cord stem cell therapy for knees is before and after x-rays. In fact, the presenter usually shows what appears to be miraculous results showing that the width of the joint has grown, suggesting that the umbilical cord therapy has caused massive new cartilage growth. Let’s dig in.
One of the big problems with using x-rays to see if cartilage has regrown is that cartilage is invisible on x-rays. Hence, you have to rely on the joint space width, which is the distance between the two bones. The problem there is that small changes in the angle of the x-ray beam can dramatically change the joint space width. For example, see my video below:
Hence, all of the x-rays I have seen from these seminars don’t actually show that cartilage has been regrown, instead, they show that the beam angle was altered to make it look like there was more cartilage.
Why Do Some Patients Report that this Stuff Works?
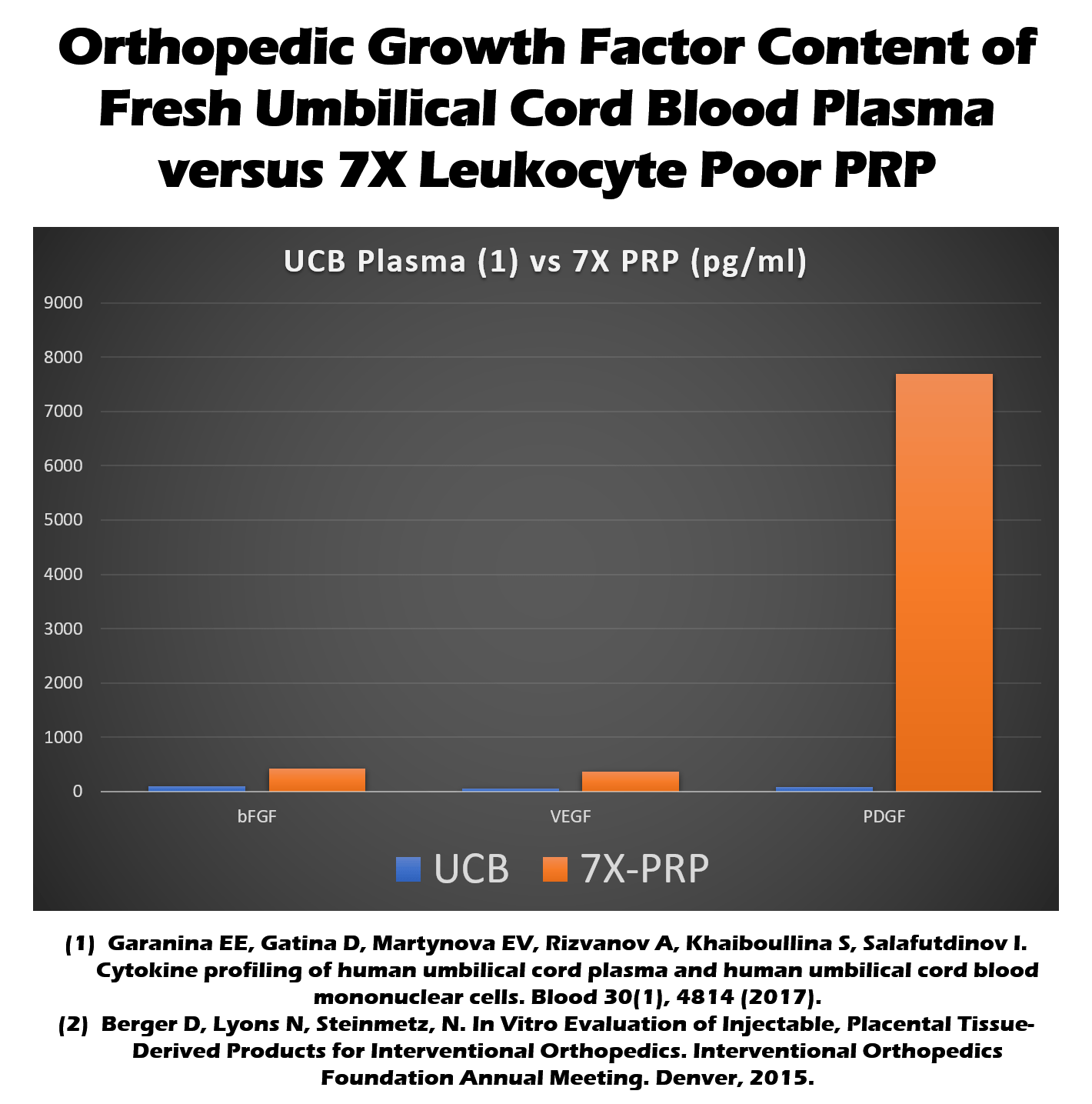
Umbilical cord preparations do contain cytokines and growth factors (12). These are molecules that can help tissue repair. How do these levels stack up against your own platelet-rich plasma (PRP) made by simply concentrating your blood platelets? As the graph below shows, your own blood platelets (7X PRP below or the red bar) turned into PRP have much higher levels of several important healing molecules than umbilical cord blood (UCB or the blue bar below).
How about the medical research evidence showing that PRP can help knee arthritis, after all, we know that there isn’t any research on how umbilical cord products can treat knee arthritis. In fact, there’s quite a bit showing that PRP can help arthritic knees (13). In fact, there are 10 published randomized controlled trials on PRP for knee arthritis with a total of 1069 patients! This research shows that PRP works.
How Much Does Umbilical Cord Stem cell therapy for Knees Cost?
The average cost for these treatments ranges from $4,000-8,000 per knee with many clinics charging tens of thousands if multiple areas are treated. Compare that to the average cost of a PRP shot for knee arthritis at around $1-2,000 and it’s not hard to see where the current value lies. Meaning we know PRP works for knee arthritis, but we have no research on umbilical cord stem cell therapy for knees.
The upshot? My deep dive into umbilical cord stem cell therapy for knees shows that we really don’t know if this stuff works. We do know it doesn’t have stem cells. We also know that many clinics are making false claims both about the stem cell content of these products, before and after x-rays, and the research showing that umbilical cord stem cell therapy works.
__________________________________________
References:
(1) Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008 May-Jun;11(3):343-53. https://www.ncbi.nlm.nih.gov/pubmed/18523506
(2) Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses. 2008 Dec;71(6):900-8. doi: 10.1016/j.mehy.2008.06.042.
(3) Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:258. Published 2015 Sep 18. doi:10.1186/s12891-015-0714-z
(4) Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014:370621. doi:
(5) Centeno C, Sheinkop M, Dodson E, et al. A specific protocol of autologous bone marrow concentrate and platelet products versus exercise therapy for symptomatic knee osteoarthritis: a randomized controlled trial with 2 year follow-up. J Transl Med. 2018;16(1):355. Published 2018 Dec 13. doi:10.1186/s12967-018-1736-8
(6) Matas J, Orrego M, Amenabar D, et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2019;8(3):215–224. doi:10.1002/sctm.18-0053
(7) Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med. 2017;6(2):613–621. doi:10.5966/sctm.2016-0157
(8) USFDA. FDA sends warning to company for marketing dangerous unapproved stem cell products that put patients at risk and puts other stem cell firms, providers on notice. 20 December 2018. https://www.fda.gov/news-events/press-announcements/fda-sends-warning-company-marketing-dangerous-unapproved-stem-cell-products-put-patients-risk-and
(9) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(10) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(11) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(12) Garanina EE, Gatina D, Martynova EV, Rizvanov A, Khaiboullina S, Salafutdinov I. Cytokine profiling of human umbilical cord plasma and human umbilical cord blood mononuclear cells. Blood 30(1), 4814 (2017). http://www.bloodjournal.org/content/130/Suppl_1/4814
(13) Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy. 2017 Mar;33(3):659-670.e1. doi: 10.1016/j.arthro.2016.09.024.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
