Utah Cord Bank Product Review

It’s always interesting to see how clinics using umbilical cord products respond to the steady stream of information that supports that these products have no live and viable stem cells. Most of the smart ones just stop making that claim, but there are a good number that just point to their suppliers who themselves continue to maintain that they are selling stem cell products. So let’s dig into one of those, Utah Cord Bank.
What is Utah Cord Bank?
This isn’t really easy to find out. There isn’t much information on their website. I was able to find about a dozen people on Linkedin that say that they’re related to the company. First up is Elliott Spencer. He has a Ph.D. in Biochemistry, but I can find no publications on mesenchymal stem cells. Outside of that, I am unable to find any other scientists at Utah Cord Bank. The most information I have found about Utah Cord Bank is actually part of this ProPublica story on Birth Tissue Sales.
What Products Do They Produce?
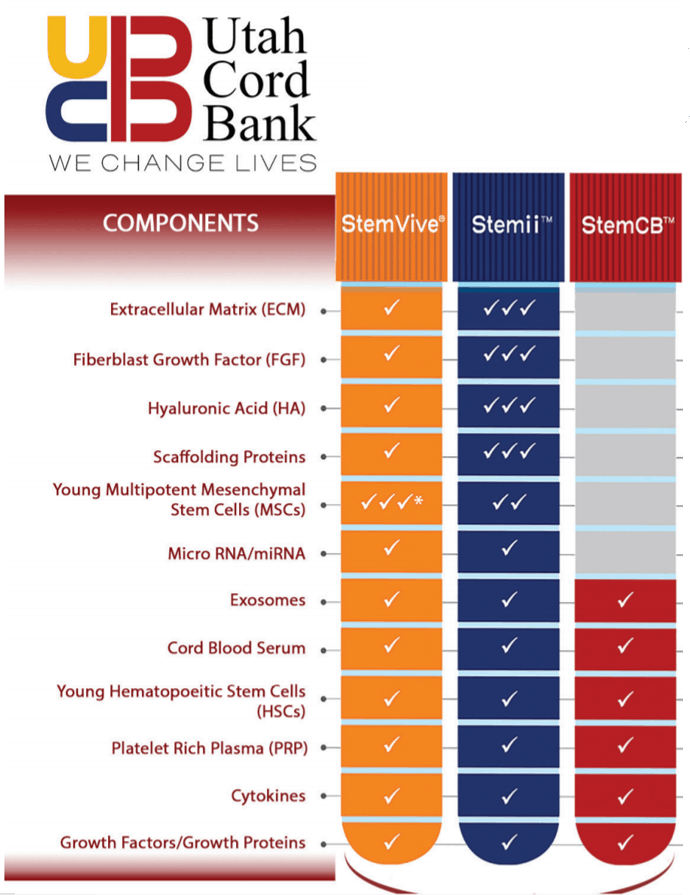
This is from the PDF listed on Utah Cord Bank’s website:
The table says that both StemVive and Stemii have Mesenchymal Stem Cells. Of those two products, the one that our lab and the lab at the CSU Translational Medicine Institute tested was StemVive, so that’s where we’ll focus.
The PDF also says this:

So Utah Cord Bank is claiming that its products can form Colony Forming Units and they are 150 times more likely to form those CFUs than bone marrow!
Can Any of these Claims Be Verified?
First, one would hope that some data would be reported on the Utah Cord Bank’s website to back up these claims. Regrettably, while you can find some scientific references that used fresh birth tissue-derived mesenchymal stem cells that were isolated and culture-expanded, none used the StemVive or any other Utah Cord Bank product. So that’s not much help.
What is StemVive?
While there is nothing on the Utah Cord Bank website on what’s in StemVive, from past investigations, this is a product made from Wharton’s Jelly. While there are mesenchymal stem cells that can be isolated from fresh Wharton’s Jelly once an enzyme is used to break down the collagen in the Jelly (4), the question is whether any of those cells survive in the StemVive product. Why? The umbilical cord, which contains the Wharton’s Jelly has to be stored and then transported to the Utah Cord Bank Lab where’s it’s likely again stored and then processed and then frozen. That vial is then sent frozen to the clinic where it’s shock thawed in someone’s hand before injection. In particular, that quick thaw method may be very hard on cells. For example, cells that are taken out of cryopreservation and used clinically are commonly recovered in culture for several days after a controlled rate slow thaw.
What’s Out There?
Since Utah Cord Bank provides no hard data to back up its claims, first we’ll take a look at what’s out there on the Internet.
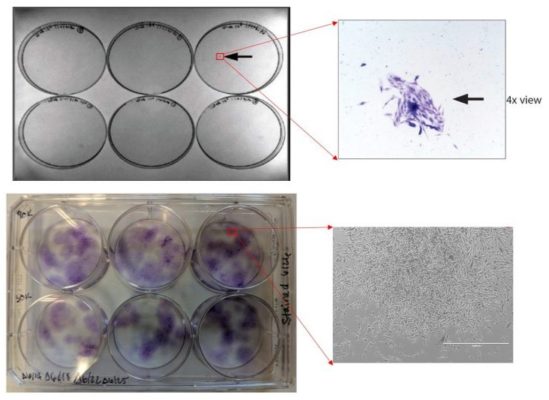
I traced the above image back to Utah Cord Bank. What is this? This is a CFU-f test that is the result of plating cells in a monolayer culture system (in this case a 6-well plate). If there are live and functional mesenchymal stem cells in the substance plated, they will adhere to the plate. You can “see” them as purple on the plates by using a stain.
So what do we see in the Utah Cord Bank plate above (on top)? A tiny speck of a stem cell colony containing a few dozen cells. For comparison, I placed actual colonies seen from middle-aged bone marrow (plate below). Notice that instead of the small speck, we see many large purple dots, each of which represents a stem cell colony of several hundred to several thousand cells.
Since I don’t have information on at what stage in the colony formation process the Utah Cord Bank product was measured, we’ll just keep this picture in the back of our minds as a placeholder. In the meantime, we have more data from actual Utah Cord Bank product that was procured for testing.
Test 1 of StemVive
We tested the Utah Cord Bank product in the same way that the literature describes it should be tested if it had mesenchymal stem cells. We took StemVive and thawed it per manufacturer instructions and then put it into monolayer culture. We also took middle-aged bone marrow and treated it the same way:
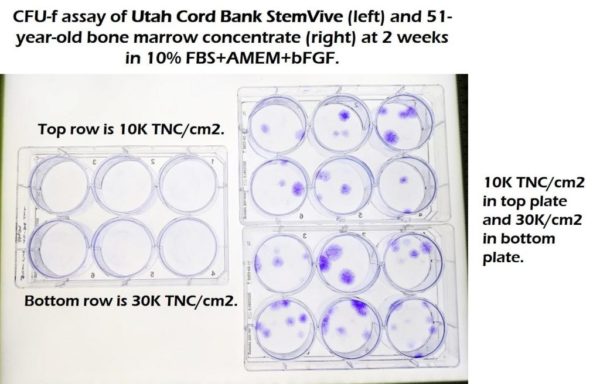
The plates on the left are StemVive and on the right is bone marrow from a 51-year-old. We see no stem cell colonies on the left and lots on the right. Hence, StemVive in this test failed to produce any viable stem cells capable of producing colony-forming units.
Test 2 of StemVive
This time we took a vial of StemVive to the CSU Translational Medicine Institute. Here are the results from that test:
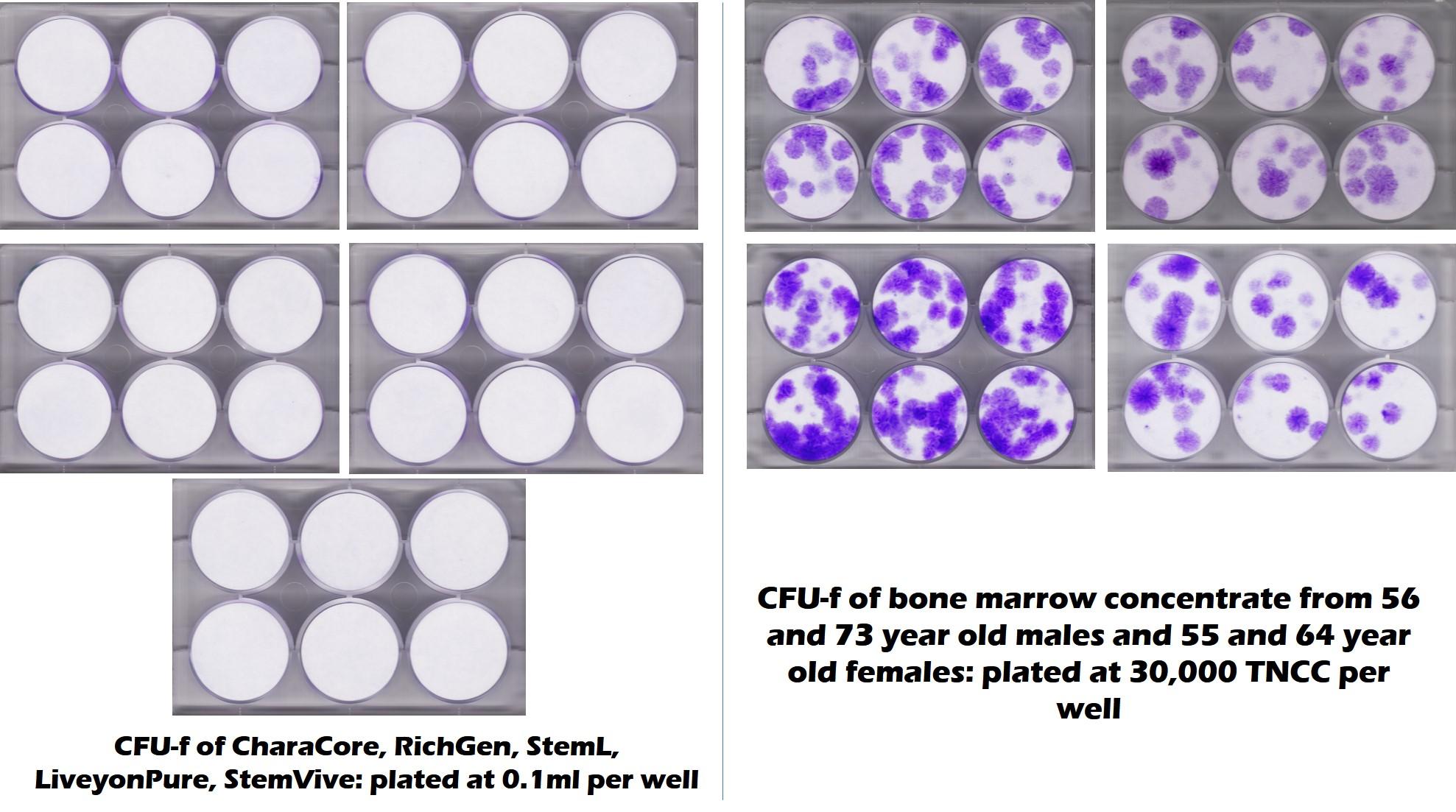
What you see above are 5 different umbilical cord products tested by CSU including StemVive on the left. On the right was bone marrow from both middle-aged and elderly donors. Again, no stem cell colonies for StemVive, but quite a few for “old” bone marrow.
My Analysis
The above two tests at two different labs are consistent with the first CFU test found online. Meaning that while StemVive or other Utah Cord Bank products may have the rare and very small CFU found, the StemVive product doesn’t generally have live and functional mesenchymal stem cells. This is also consistent with others who have tested other birth tissue products and published or presented the results at scientific conferences (1-3). Hence the assertion that StemVive has 150X the colony-forming capabilities of bone marrow isn’t supported. In fact, you might be able to make that argument in reverse in favor of bone marrow.
The upshot? It’s time for Utah Cord Bank and all umbilical cord and amniotic product vendors to begin to own up that they are not actually selling products with any live and functional mesenchymal stem cells. My opinion is that the reason why they haven’t is that their pricing and sales models are now tied to that assertion and giving it up could mean financial ruin.
__________________________________________________
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Marino L, Castaldi MA, Rosamilio R, et al. Mesenchymal Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential. Int J Stem Cells. 2019;12(2):218–226. doi: 10.15283/ijsc18034

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.