What Is the Stem Cell Machine?
One of the more interesting aspects of the increasingly dangerous stem cell wild west is the provider and patient exploitation that is occurring because of misinformation. Today I’d like to highlight what should just be legally sold as a shockwave device, but instead, a chiropractor in Atlanta is selling it as “The Stem Cell Machine”. Let’s dig in.
What Is “The Stem Cell Machine”?
So what is this? Turns out this is a simple ESWT machine using decades-old technology and being resold by a chiropractor by the name of Matt DiDuro.
What Is ESWT?
ESWT stands for Extracorporeal Shock Wave Therapy. This technology is a half-century-old as it was first used in 1971 (when I was in third grade) to treat kidney stones. It works by creating a high-intensity shockwave that injures the tissue. For orthopedic problems, this technology works much in the same way that prolotherapy injections create a microinjury to prompt healing.
We’ve never used it because it’s like using a sledgehammer to put in a finish nail. Meaning, there is no way to direct this energy to specific spots, the best you can do is to create a microinjury to a region. So the sharpshooter technique we usually use to inject orthobiologics into the specific areas of injury is replaced with a shotgun approach.
Does ESWT Work?
For the most part, there is good data that ESWT works to help tendon and fascial injuries that are easily accessible. Meaning, for something like plantar fasciitis, where the plantar fascia is superficial and can easily be accessed with sound waves, ESWT takes about 6 treatments to get an effect. That compares to the efficacy of a single ultrasound-guided PRP injection to get the same effect.
However, for something like an ACL tear where the ligament is buried deep inside the bone of the knee, ESWT is not going to be as effective. Why? It’s difficult to bounce sound waves into the knee when there is bone is the way. The same would hold true with most spinal problems.
Is a Half Century Old Technology a “Stem Cell Machine”?
All orthopedic tissue healing involves local progenitor cells or mesenchymal stem cells. So if you go out and sprain your ankle, it’s the local stem cells that help heal those damaged ligaments. If a doctor injects prolotherapy solution to cause a micro-injury in those nonhealing ligaments, it’s stem cells that help heal that injury. If you apply an infrared heating machine at home to improve blood flow and cellular activity in those sprained ligaments, again, it’s stem cells that heal that injury.
So while ESWT healing also involves stem cells, calling this a “Stem Cell Machine” is a HUGE stretch. That would be like saying that the infrared unit we sell in the office is a “Stem Cell Machine”. Or that deep tissue massage or Rolfing, since these both cause some local tissue damage, are stem cell therapies.
Reaching out to Dr. DiDuro
In my quest to find out more about this device, I booked an appointment to speak to a sales rep. I got both the rep and the chiropractor who is selling the device, Matt DiDuro, DC of Atlanta. I asked some hard-hitting questions and here’s what I learned:
1. Matt told me that since there are animal studies that show that ESWT activates stem cells, then he feels that he should be able to call this a stem cell machine.
2. When I brought up that this name changes the regulatory classification of this device from a simple 510K clearance to a PMA, he was confused by these terms.
3. He told me about all sorts of clearances by the FDA for all sorts of indications including “tissue regeneration” and then told me that he would send me the FDA documentation. All I got was a simple 510K clearance for the use of the machine to help diabetic foot ulcers (see below):
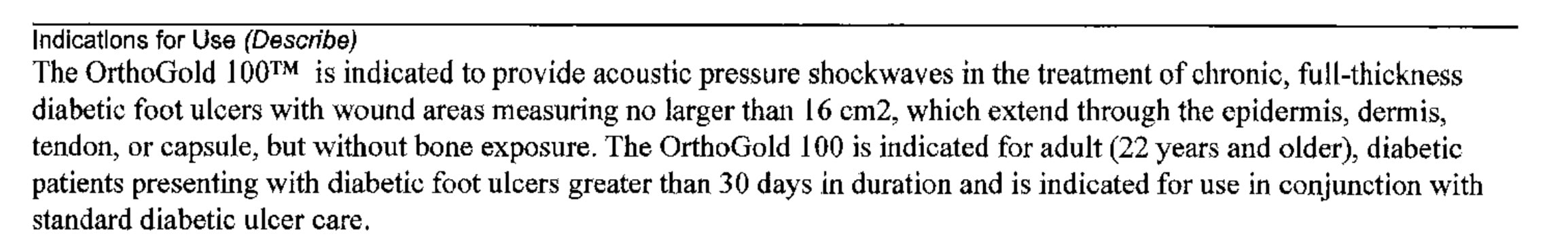
In fact, the only reference to “tissue regeneration” in the entire document is the reference to “Tissue Regeneration Technologies” which is a company where Dr. DiDuro sits on an advisory board and that submitted the 510K.
Does the FDA Know that this Device Is Being Marketed as “The Stem Cell Machine”?
NO. There is no mention of uses like tissue regeneration or stem cells in the FDA 510K clearance, which is focused on a device to treat diabetic foot ulcers. To understand why that’s critical here, you need to learn a little something about how FDA device approvals work.
The easy way to get a device to market is a 510K clearance. This process is quick and relatively cheap. Meaning an experienced company can get one in about 6 months with a small business filing fee of $3,108. All in, the cost is usually on the order of tens of thousands to get everything together to submit the application.
A 510K clearance is all about pointing to a predicate device that is similar to yours and was usually grandfathered in or has received FDA approval. Hence, the only thing you need to prove to the FDA is that your device is “substantially similar” to the device they accept. Meaning the FDA is not really looking at any clinical data on how your device does or doesn’t work. This is why this is such a fast and cheap process. The downside? This process dramatically limits what you can say about the device’s efficacy.
On the other side of the FDA approval coin is a PMA, which stands for Premarket Approval. Hence if Dr. DiDuro wanted to claim that this device works by activating stem cells, like this statement on his website:
“Immediately following treatment there is typically a significant reduction in pain, swelling and inflammation, as well as improved range of motion. Four to eight weeks after treatment there is an increase in blood vessels and circulation, which supports faster healing of tendon and bone. And for up to twelve weeks following a treatment there is a recruitment of stem cells to the treated area for the regeneration and healing of tissues.”
He would need to file for a PMA. That requires a filing fee of almost 100K for a small business and requires clinical trials to prove the statement. It would take at least 2-3 years to perform the studies needed to prove this statement plus likely tens of millions of dollars.
More Exaggeration?
So have these academic medical centers like Harvard or Mayo Clinic approved the use of their logos or do they endorse “The Stem Cell Machine”? Nope, not according to DiDuro. These are simply places that own machines.
Misbranding?
Misbranding simply means that a manufacturer is claiming something that he or she is not permitted by the FDA to claim. IMHO, all that Dr. DiDuro is permitted to claim is that this ESWT device can be used to treat diabetic foot ulcers. Stop, do not proceed any further. So calling this a “Stem Cell Machine” with the description above, IMHO would be misbranding. In fact, merely pointing to the FDA 510K clearance above (which is done twice), may cause problems for Matt based on this statement brought up in the 510K registration letter provided to me:
Sec. 807.39 Misbranding by reference to establishment registration or to registration number.
Registration of a device establishment or assignment of a registration number does not in any way denote approval of the establishment or its products. Any representation that creates an impression of official approval because of registration or possession of a registration number is misleading and constitutes misbranding.
Misbranding is some serious stuff and this is what the law says about that:
“Section 331 involving a mislabeled consumer product other than certain drugs is punishable as a misdemeanor, carrying a maximum sentence of one year in federal prison and/or a $1,000 fine (21 U.S.C. § 333(a)).”
The upshot? Is this really a “Stem Cell Machine? Not IMHO. In addition, while it was represented to me that the FDA cleared the device for “tissue regeneration”, no such documentation exists based on what I was sent. In addition, describing this device that way may carry serious consequences. As I always say, you can’t make this stuff up!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.


