Do NP’s or PA’s Have Liability for Injecting Umbilical Cord Stem Cells?
I get pinged by health professionals all the time. This weekend I got an email from a nurse practitioner who was concerned about her legal liability in offering an umbilical cord “stem cell” product. This is such a complex issue, I told her I would blog on it without using her name.
What is an NP or PA?
Nurse Practitioners (NPs) or Physician’s Assistants (PAs) have more training than a nurse, but not as much as a physician. For example, most have about a quarter to half the training of a physician specialist. In some states, they can practice more independently and in some states less independently. These professionals serve an important role in the healthcare delivery system, often working alongside physicians. Hence, a medical practice with four doctors may also have four PAs or NPs who also see patients who require less attention from a physician. In rural settings, there may be no physician and only a nurse practitioner.
The Rise of “Integrative Health” Clinics
Over the last decade, we’ve seen more alternative health offices like chiropractic, naturopaths, and acupuncturists hire NPs and PAs to perform injection or other types of care their licenses don’t authorize. This is an arrangement that can create some inherent problems. Let’s dig in.
When NPs/PAs are employed in a medical practice with physician specialists, they can easily bounce ideas and plans off the physicians in that practice, they can also transfer more complex patients to a physician when they’re not comfortable. In addition, only the lower level procedural care would get transferred to a NP/PA with the physicians performing the procedures that are higher risk. In a rural setting, NPs are often practicing independently and tasked with well known primary care tasks such as well checks/annual exams, prescribing medications for routine diseases, and small office procedures like placing or removing stitches.
This dynamic changes in alternative care practices. Now the NP/PA is the one with the most medical knowledge, as the chiropractor, naturopath, or acupuncturist has much less training in recognizing and treating sick people. Meaning only the NP/PA was trained in a university setting with many sick patients. This exposure to what to do when someone gets really sick and how to identify who needs much more care is a critical skill.
Birth Tissues, Integrative Health Clinics, and NP/PAs
The Liveyon debacle, where bacterial contaminated umbilical cord blood was sold to clinics and injected by both physicians and NPs, and PAs is now defining the legal exposure these professionals face by offering “stem cell” therapy (9). How do I know? I’m the medical expert on most of these cases.
This risk to NP/PAs by participating in “stem cell therapy” using birth tissues can be divided into three categories:
- Fraud
- Informed consent
- Treatment below the standard of care
Understanding these issues is critical for NPs and PAs who perform these injections and who expect to keep their licenses to practice.
Fraud
First, as you know from reading this blog, we now have the results of multiple research-based investigations that show that amniotic and umbilical cord tissues have no living and viable stem cells (1-3). Those studies will soon have a new edition as we submit for publication our joint project with a university which was an investigation into five commonly used birth tissue products that had no living stem cells. The image below clearly shows no growth in monolayer culture in these products (left) while the right shows stem cell growth in middle-aged and elderly patient’s bone marrow:
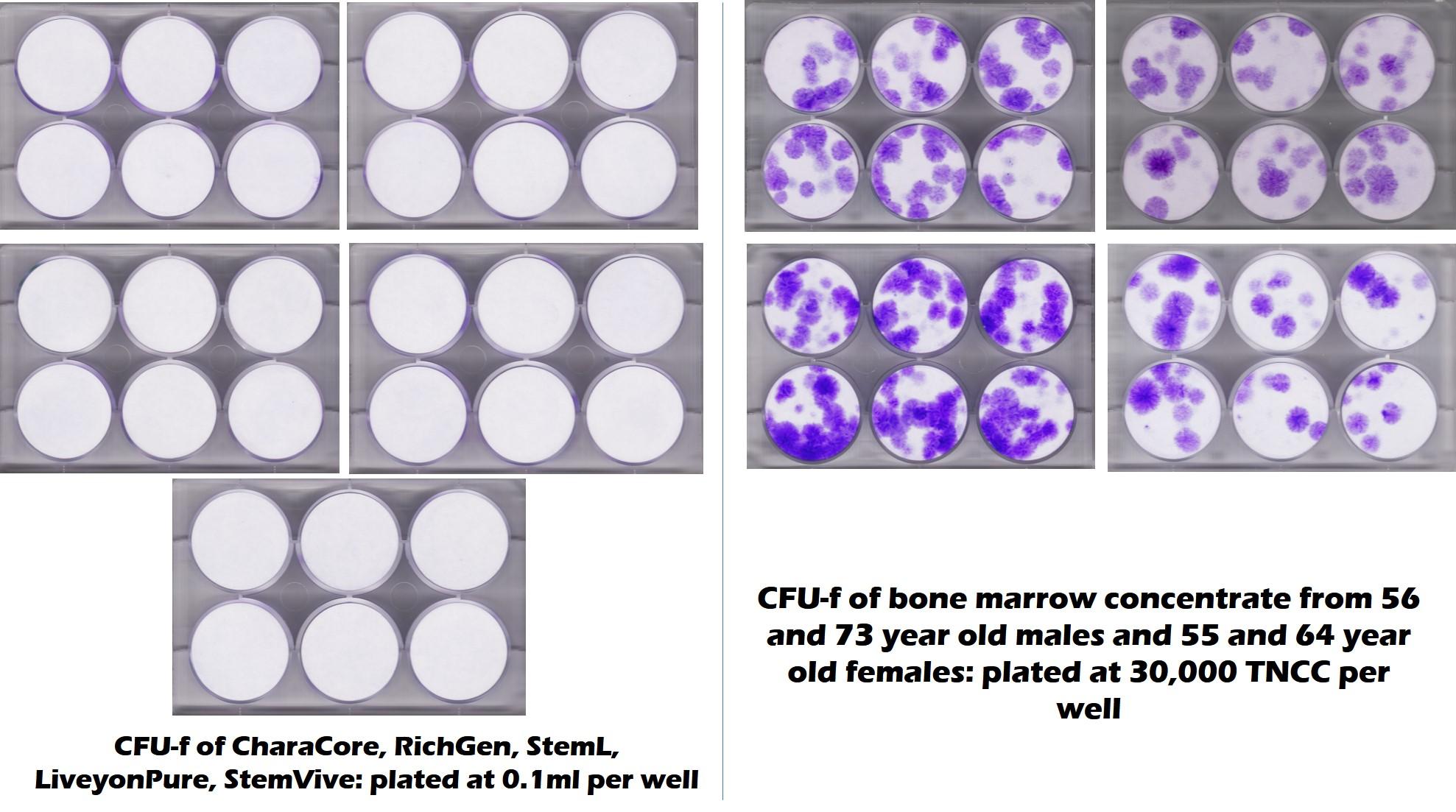
Why is all of this critical if you’re a Nurse Practitioner or Physician Assistant? If you claim to a patient that you are injecting your patients with an amniotic or umbilical cord product that has millions of young and viable stem cells because their bone marrow cells are just too few, that’s fraud based on the existing published and presented studies. Both Nurse Practitioners and PAs have false claims provisions in their practice acts, hence a complaint here could earn them everything from censure to a restricted license, to loss of license, depending on the offense and the state. In addition, if there is a complication that leads to a malpractice case, exhibit A in that case, will be the fraud involved in convincing the patient to proceed with the therapy.
Informed Consent
Informed consent in medicine protects patients and medical providers. On the patient side, you should get an accurate description of the procedure and its risks. On the provider side, you have discussed with the patient the risks and he or she has decided to sign off (or not) that they accept those risks and want to proceed.
However, an informed consent document for amniotic and umbilical cord products can’t be accurately written. This is because the research on human risks when these products are used in an integrative clinic is largely absent. Let’s dive in deeper here.
NPs/PAs that have spoken to sales reps will get the song and dance that these products have been used hundreds of thousands of times without any known complications. In fact, patients get that same song and dance at seminars. However, we know that’s not close to true. Let’s run down just two common delivery routes: IV and intra-articular. These are both common practices in integrative health clinics.
We know for example that HLA typing is required for the intravenous use of umbilical cord products (4). For example, in real-world medical settings like pediatric cancer care where umbilical cord blood is used, doctors perform tests on individual lots of umbilical cord blood to find out the type of HLA markers on these cells and how similar to or different they are from the planned patient. The closer the match, the less the chance there is of rejection (5).
Hence, any informed consent for a patient receiving umbilical cord blood would need to state that since the provider is not HLA matching the blood to the patient, there are potentially serious risks. In addition, this would need to be explained to the patient that the side effects could include a skin rash, organ failure, or death. How many patients get this explained right now? I have never met a single one.
On the unknown risk side, let’s take a knee injection of umbilical cord blood. Right now, in the US National Library of Medicine, there are only 13 entries for “umbilical cord blood knee osteoarthritis” and these represent the clinical results of 9 patients (6). Not a single one of these studies use any commercially available umbilical cord product, but instead isolated and culture-expanded stem cells from fresh umbilical cords. Meaning, whatever safety data that exists in this handful of studies can’t be used for what the NP/PA is injecting, as all of the data shows that the mesenchymal stem cells used in these products are dead. In addition, commercially available umbilical cord preps have loads of nucleated cells and other tissue not found in the injectate used in these studies.
In summary, whatever consent the NP or PA uses here is a malpractice suit waiting to happen. Meaning, there is no way to ethically consent the patient when performing amniotic or umbilical cord injections. Which brings us to the next topic, which is that of medical research.
Treatment Below the Standard of Care
Any treatment you offer a patient has to have a reasonable expectation of benefit. In established therapies that benefit can be based on both research and the group experience of millions of medical providers over decades. In fact, the whole risk/benefit ratio is based on the idea of benefit. For example, if the benefit is huge and the risk is major, then the procedure may be worth the risk. However, if the benefit is small or unknown and the risk is big, then the procedure may not be worth performing. Which brings us to our next topic, medical care versus research.
As discussed, if the patient gets injected with a commercially available umbilical cord tissue to treat knee arthritis, we have no research on benefit. We also have no data on risks. In addition, this is a new therapy for this application, so no long-term medical community data exists. If you focus on injecting these products IV, again there no data on risks or benefits. Hence, if there’s a complication and the provider can’t show a reasonable expectation of benefit, the use of the product is malpractice per se.
In fact, the only way to avoid these issues is through an institutional review board (IRB) and offering medical care as part of medical research. In this case, if we look at two seminal bioethics documents on the topic of medical care and research, they state:
Belmont (7):
“When a clinician departs in a significant way from standard or accepted practice, the innovation does not, in and of itself, constitute research. The fact that a procedure is “experimental,” in the sense of new, untested or different, does not automatically place it in the category of research. Radically new procedures of this description should, however, be made the object of formal research at an early stage in order to determine whether they are safe and effective.”
“Research and practice may be carried on together when research is designed to evaluate the safety and efficacy of a therapy.”
Declaration of Helsinki (8):
“In the treatment of an individual patient, where proven interventions do not exist or other known interventions have been ineffective, the physician, after seeking expert advice, with informed consent from the patient or a legally authorised representative, may use an unproven intervention if in the physician’s judgement it offers hope of saving life, re-establishing health or alleviating suffering. This intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available.”
Hence, you can use these tissues as part of medical practice combined with research, but you had better collect that data using validated questionnaires as part of a treatment registry and publish your results. However, this is NOT what’s happening. Hence, any complication that occurs using these tissues would be malpractice per se, as there is no benefit and the procedure was not part of formal research.
Regulatory Issues
In addition to all of the risks above, yet another issue is that there can be major regulatory problems when a provider claims that these are stem cell products. That gets even worse when the provider is part of a lecture, advertisement, or website making that claim. Why?
The FDA has made it clear these birth tissue products that claim live cells should be approved through the cell drug regulatory pathway (9). This is not because they have any live cells, but merely the claim of live cells makes them a drug. Meaning, the regulatory status of what you’re selling is determined more by the claims you make than what you sell. However, the manufacturers of these products have incorrectly (and illegally) registered them as a 361 tissue (10). This is not an FDA cell drug approval as is required, but merely a free 45-minute quickie registration that amounts to “tell us what you’re selling and where you live”. Hence, these products are by definition adulterated and misbranded drug products.
What happens to medical providers who promote adulterated and misbranded drugs? Specifically, the Food, Drug, and Cosmetic Act in section 301 informs us that it is illegal to distribute or promote a drug product that is adulterated or misbranded. Meaning you can end up in federal prison for promoting umbilical cord “stem cell” treatments.
The Intangibles
Right now, “stem cell” therapy and birth tissues are a highly charged and sensitized topic. Medical and nursing boards are getting pressure from the FDA, FTC, and FMSB to crack down on clinics (11-13). Hence, having a patient who suffers complications or gets no results despite paying thousands of dollars and that generates a board complaint is problematic. Add to that that there was no data supporting the safety or efficacy of the injection you performed and you may have a perfect storm for losing your license.
The upshot? If I were an NP or PA, I would steer clear of amniotic and umbilical cord stem cell use. Unless you’re using these products as part of an IRB approved study and have the intent of publishing the data, your license to practice is on the line as well as your malpractice policy. So if a few extra thousand dollars a year worth not having a license?
________________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Eapen, Mary et al. Mismatched Related and Unrelated Donors for Allogeneic Hematopoietic Cell Transplantation for Adults with Hematologic Malignancies. Biology of Blood and Marrow Transplantation, Volume 20, Issue 10, 1485 – 1492. https://www.ncbi.nlm.nih.gov/pubmed/24862638
(5) Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. https://www.ncbi.nlm.nih.gov/pubmed/15191952
(6) US National Library of Medicine-PubMed. Search on “umbilical cord blood knee osteoarthritis> Accessed 25, August 2019. https://www.ncbi.nlm.nih.gov/pubmed/?term=umbilical+cord+blood+knee+osteoarthritis
(7) United States. (1978). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research. Bethesda, Md.: The Commission. https://www.hhs.gov/ohrp/sites/default/files/the-belmont-report-508c_FINAL.pdf
(8) World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191-4. doi: 10.1001/jama.2013.281053.
(9) US Food and Drug Administration. “FDA sends warning to company for marketing dangerous unapproved stem cell products that put patients at risk and puts other stem cell firms, providers on notice” 12 August 2019, https://www.fda.gov/news-events/press-announcements/fda-sends-warning-company-marketing-dangerous-unapproved-stem-cell-products-put-patients-risk-and .
(10) US Food and Drug Administration. “Tissue Establishment Registration” 11 August 2019, https://www.fda.gov/vaccines-blood-biologics/biologics-establishment-registration/tissue-establishment-registration.
(11) US Food and Drug Administration. “Statement by FDA Commissioner Scott Gottlieb, M.D., and Biologics Center Director Peter Marks, M.D., Ph.D. on FDA’s continued efforts to stop stem cell clinics and manufacturers from marketing unapproved products that put patients at risk” Accessed 25 August 2019, https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-biologics-center-director-peter-marks-md-phd-fdas
(12) Federal Trade Commission. “Stemming unproven stem cell therapy claims”. Accessed 25 August 2019. https://www.ftc.gov/news-events/blogs/business-blog/2018/10/stemming-unproven-stem-cell-therapy-claims
(13) Federation of State Medical Boards. “FSMB RELEASES RECOMMENDATIONS ON REGULATING PHYSICIANS’ USE OF STEM CELL AND REGENERATIVE THERAPIES”. Accessed 25 August 2019. http://www.fsmb.org/advocacy/news-releases/fsmb-releases-recommendations-on-regulating-physicians-use-of-stem-cell-and-regenerative-therapies/

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
