CFU-f 101: To Be an Orthobiologics Expert You MUST Learn This
The blind leading the blind is one of my favorite expressions. As one of the founders of this field of interventional orthobiologics, the fact that this statement applies to much of our existing orthobiologics education is concerning to me. Nowhere is this more true than the concept of stem cell dose and CFU-f assays. Hence, this morning let’s dive into this topic.
The Game of Telephone
There’s a saying in medicine, “See one, do one, teach one”. Meaning, in medical school, internship, and residency, there’s a lot to learn, so that uptake had better be quick. While that may sound scary that doctors are educated in that way, when you have strict educational criteria where student physicians are the brightest of the brightest and you surround them with a tough didactic curriculum and then wrap that in formalized hands-on education, it kind of works.

Credit: Shutterstock
However, there is no such standard curriculum in orthobiologics. While I founded an organization called IOF that is slowly but surely extending a formalized curriculum throughout this space, most orthobiologics education is still offered by commercial companies and what you get taught depends on the guy teaching. That’s also influenced by the telephone game. Let me explain.
Do you remember that game we played as kids? You take a statement and pass that secretly to the person next to you and then that person passes it to the next person and so on. By the time that very clear statement gets to the last person, it tends to be garbled. That’s what I see happening in orthobiologics education. The first-generation physicians had to learn much more of the basics of cell biology to be able to offer procedures that were firsts in the field. There we no kits, no research papers, no textbooks, no organizations or conferences, so they became self-taught. The second generation also had to know some of that, but not most of it. By that time there were at least kits and some research. The third and fourth-generation physicians got to use idiot-proof kits, went to conferences, had research to fall back on, and new textbooks. They also got much of their education largely from manufacturers.
One of the areas where this lack of basic knowledge shows up is in cell biology. To provide an example, today we’ll dive into a basic concept that every person in that game of orthobiologic telephone had better understand, “What is a CFU-f?”
What Is a CFU-f?
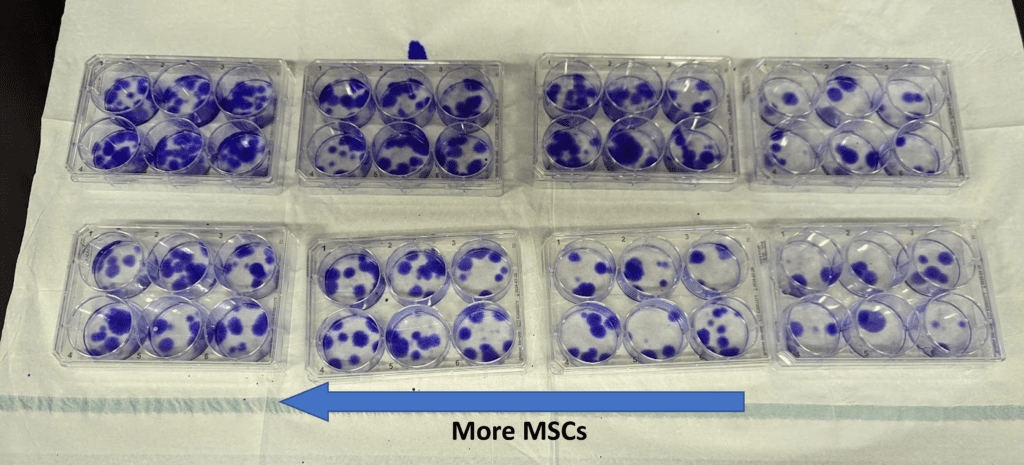
A picture is worth 1,000 words. See those purple dots on those 6 well culture plates? These are from our in-house lab and each dot is a colony of adherent cells that formed when bone marrow concentrate was plated and incubated. The plates on the left have a higher CFU-f count and the ones on the right have a lower count.
CFU-f stands for “Colony Forming Unit-fibroblast”. It’s critical to understand because it’s the basic unit of stem cell dose in all advanced orthobiologics like bone marrow concentrate or Mfat. In addition, increasingly, this stem cell content has been tied to outcomes. Meaning if the sample you inject has more CFU-f’s, it has more mesenchymal stem cells and is more likely to be effective and associated with a better patient outcome (1,2). Hence, making sure that the technique with which you harvest and then process tissue produces the absolute highest CFU-f counts possible is critical for maximizing the chances of patient success.
Our research team has published several papers using CFU-f assays including one just accepted for publication and not yet out in print (3). Hence, as medical director of that research team, I’ve lived all of this in real-time as part of my day job. I have learned all of this the hard way from the experts on our scientific team.
First, this is not an exhaustive list, but rather enough detail so that you can see a fake CFU-f count from a mile away. A basic CFU-f assay is performed by:
- Processing the tissue to isolate nucleated cells
- Pre-preparing the sample (usually to lyse or get rid of red blood cells)
- Counting the number of nucleated cells you have
- Diluting those cells into a few different dilutions (i.e. 5,000 cells/ml, 10,000 cells/ml, and 20,000 cells/ml)
- Plating each dilution in a monolayer culture flask usually in duplicate or triplicate
- Adding “cell food” to that culture flask (AMEM/DMEM (nutrients) + growth factor rich media (FBS or platelet lysate))
- Incubating these flasks under standardized conditions for 7-10 days
- Once colonies shave formed, staining these flasks so that the colonies are easily visible
- Counting these colonies using a standardized technique
- Deciding which of your dilutions maximized your CFU-f count and then using that count
CFU-f Variables
While CFU-f is a great way to study the dose of stem cells in a research study where the results obtained by one lab are used to compare technique A vs B or to determine if that metric corresponds with clinical outcome, the assay has way too many variables to be more generally useful as a dose metric that can be compared between studies or labs. Why? Let’s look at all of the variables that go into this assay:
- Processing the tissue to isolate nucleated cells — There are many different ways to process bone marrow or Mfat which all have different efficiencies in concentrating MSCs.
- Pre-preparing the sample — If you get rid of the RBCs, this usually increases the CFU-f count. However, not every lab adds this step.
- Counting the number of nucleated cells you have — While this may seem simple, we have commercial labs out there being used by researchers that don’t include this step. Even when it’s done, there are several ways to count nucleated cells that give different numbers in the same patient.
- Diluting those cells into a few different dilutions — This is done because how many cells you plate determines how many colonies you get, often in an inverse relationship. For example, lower plating densities often produce more colonies than higher plating densities. However, not every lab even uses this step. For example, I know of at least one commercial lab being used by researchers that usually skips it altogether. Others may only use one dilution, which is a problem, as the plating density directly impacts the CFU-f count. That’s also an issue because depending on the number of MSCs in your samples, you may be undercounting CFU-fs.
- Plating each dilution in a monolayer culture flask usually in duplicate or triplicate — This is critical as you need verifiable results, so having several identical samples on the same patient allows you to know that your lab procedures are producing reliable and reproducible results. However, again, I know of commonly used commercial labs that again skip this step and just plate one sample per patient as that’s less work in the lab.
- Adding “cell food” to that culture flask — There are lots of choices here that all influence that final CFU-f number and many labs do this differently. AMEM or DMEM? FBS (Fetal Bovine Serum) or PL?
- Incubating these flasks under standardized conditions for 7-10 days — Here, your altitude matters! A lab growing cells in Denver or Vail that doesn’t control the oxygen content of the incubator will produce different CFU-fs than one at sea level. Also, you can grow cells in incubators that remove oxygen (hypoxia) and this also changes the final CFU-f number.
- Once colonies have formed, staining these flasks so that the colonies are easily visible and then counting these colonies using a standardized technique — If you think that we have too many variables to keep track of above, you’re about to get truly dizzy with this step. Here, how you count these colonies is critical. The problem is that many times colonies will form close together or overlap. Look closely at the image above from our lab of CFU-f 6 well plates. In some wells you have to ask yourself, is that one colony or two? In addition, some colonies are large and easily counted and others are tiny and may not be counted as a colony. In fact, in our lab, at one point we had three different people reading the colony number just for grins and found that one reader always counted high while another was always more conservative. There are also different computer programs that can do this, but they all count differently and all have various levels you can set that will change the final count. Hence, only one reader, using strict criteria, should ever count these plates and several counts should be performed on each sample for accuracy.
- Deciding which of your dilutions maximized your CFU-f count and then using that count —The reason you plate at multiple cell dilutions is because different patient samples will vary widely in their MSC content. Hence, many times the 5K/ml dilution will show a maximum CFU-f count in one patient while the 20K/ml dilution will show that max count in another. As above, many commercial labs skip this step altogether, rendering the CFU-f count useless.
CFU-f Confusion and My CFU-f’s Are Better than Yours!
I have seen more third and fourth-generation orthobiologics physicians who are now performing research get on the podium at various conferences and botch basic ideas around CFU-f’s than I care to mention. One crazy example is the statement that their BMA technique or the machine or device that they use produces X CFU-f’s which is more than someone else’s reported CFU-f counts in a white paper or published research. I hope at this point, now that you know the crazy number of variables involved that you can plainly see that this statement demonstrates that the researcher knows enough about CFU-f assays to be dangerous. YOU CAN NEVER COMPARE CFU-f COUNTS BETWEEN LABS!
Your Responsibilities as a Researcher
Let’s say, that because you don’t have an in-house research lab and PhD-run lab team in your practice, you decide to farm out your CFU-f counts to a commercial lab for hire. You then send samples and get CFU-f counts back and then incorporate those into a research paper that is eventually published. It’s your responsibility as a physician-researcher to know if those counts are accurate or if the CFU-f assay was botched or poorly done. What would happen if at some later date it was revealed that the commercial lab did these counts wrong? You’re potentially liable for academic fraud, meaning it was your responsibility to know the difference and sniff out a bad result and poor lab techniques.
The upshot? You can’t be an expert in orthobiologics on the podium lecturing and teaching other physicians without knowing the basic cell biology behind your data or your topic. So here’s a challenge to all of those second, third, and fourth-generation doctors in the game of orthobiologics telephone. If you don’t understand CFU-fs well enough to walk a lab tech through how it should be done, then please learn!
_______________________________________________________
References:
(1) Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015 Jan;33(1):146-56. doi: 10.1002/stem.1845. PMID: 25187512.
(2) Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002 Dec;(405):14-23. doi: 10.1097/00003086-200212000-00003. PMID: 12461352.
(3) Berger DR, Aune ET, Centeno CJ, Steinmetz NJ. Cryopreserved bone marrow aspirate concentrate as a cell source for the colony-forming unit fibroblast assay. Cytotherapy. 2020 Sep;22(9):486-493. doi: 10.1016/j.jcyt.2020.04.091. Epub 2020 Jun 19. PMID: 32565131.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
