Direct Biologics and Kimera Response on Exosomes: Confusing Physicians 101
If you were making big bucks selling exosome products, December of 2019 was a very bad month. The FDA came out with a rare public warning on exosome products that basically destroyed your business plan. Or did it? Is there a way you can easily recover? Sure, confuse the heck out of doctors by throwing a dense set of regulatory terms at them. Let’s dig in.
What Are Exosomes?
If you haven’t heard about exosomes before, they’re little vesicles secreted by all cells that act as a form of cell to cell communication. They are basically the words and phrases that cells use to speak to one another. The idea that we have any idea how to create a reliable exosome product for sale by isolating only the words and phrases that have to do with tissue repair is frankly ridiculous. To understand more about exosomes, take a look at my video:
The FDA Warning on Exosomes
If you’re a watcher of the stem cell and exosome wild west, you know that the FDA can be slow to act. However, in December 2019, the agency acted with lightning speed to get ahead of the exploding space of companies claiming to sell exosome products to treat everything from penile dysfunction to neurologic problems, to aging. They issued this rare public warning:
This warning is very clear, no matter how you cut it, EXOSOMES ARE A DRUG PRODUCT that requires FDA APPROVAL BEFORE HUMAN RESEARCH OR USE.
So if you are were selling these products and making big bucks, what can you do? You either close up shop and invest tens of millions in an FDA approval and wait 3-5 years per clinical indication or you chance it and throw down a smokescreen to confuse doctors. It’s this second strategy that I’ll be covering today, as for the most part, near as I can tell, the smokescreen seems to be working with gullible physicians, while the doctors that know this space aren’t biting.
Understanding the FDA Regulatory Process
Before I dive into what are, in my opinion, two masterful attempts by exosome companies to confuse doctors, let’s review how the FDA process works, as this is the first place where doctors get really bewildered. Unlike anything else doctors come in contact with, the claims you make when selling FDA regulated products drive the regulatory paradigm or pathway. The example the agency gives is charcoal.
If you sell charcoal for retro backyard grills, then what you’re selling is not FDA regulated. However, if you take the same exact product and put it in a bottle and start selling it to treat poisoning, it becomes FDA regulated as a drug. Do you see the difference? Merely a claim that your charcoal can treat a disease is what requires FDA approval.
Hence, what the FDA warning on exosomes is saying first and foremost is that if you’re selling an exosome product that you claim can treat a disease–it’s a drug. Hard stop. Do not pass Go. So no matter how much wrangling you try after that to creatively fit your product into some sort of fabricated exception or bizarre loophole, it will make no difference. A bit like trying to put the pin back in a hand grenade in the cartoons, it never seems to work out well.
The 361 Tissue Dodge
Instead of getting an FDA drug approval (351 drug pathway) which takes tens of millions and many years, all exosome manufacturers perform a free, quickie 45-minute tissue registration known as a “361”. This pathway is actually for selling tissues, not products manufactured by growing cells in culture. Hence, any company that is manufacturing exosomes in a lab that uses it is merely applying the wrong FDA pathway.
The 361 tissue registration process is actually called “Tissue Establishment Registration“. You basically fill out an online FDA form that tells the agency what you’re selling and where you can be found and a few other details and you’re good to go. There is NO FDA approval or even review process. They do reserve the right to visit your lab to make sure you’re using sterile technique and good tissue handling and screening practices.
Direct Biologics
While researching another blog, I ran across the Direct Biologics response to the FDA public warning. In my opinion, it’s frankly a master class in how to confuse physicians. Let’s dig in.
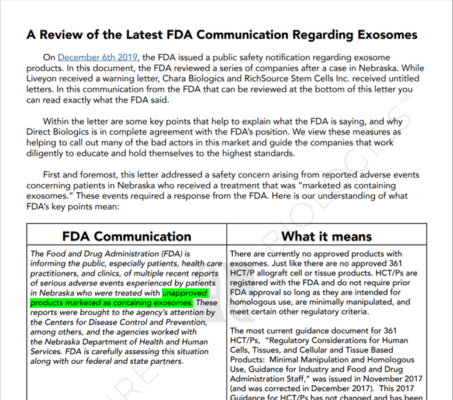
First, Direct Biologics is a medical distributor who has been selling exosome products for the last several years. Second, let’s look at what they claim on their website:
So they claim that they sell exosomes. They also state, “The ExoFlo exosomes are isolated from donated human mesenchymal stem cells (MSCs) and purified using proprietary processing.” There are a host of claims after that:
- Exosomes help tissue regenerate
- The optimal way to perform “stem cell therapy” is to deliver the exosomes created by stem cells
- Their exosomes can activate and recruit the patients’ own cells to the area, revascularizing the area and reducing the inflammatory response to allow natural healing.
The company also has a host of videos on YouTube. Suffice it to say that, in my opinion, these claims of healing are enough to meet the definition of a treatment claim. Hence, since the company is claiming to sell exosomes and attaches treatment claims to that product, the direct biologic product is a 351 drug requiring full FDA approval, which it does not have.
So what has the company put out there as a smokescreen? This document:
In my opinion, this thing is a mixed-up mess of how to confuse physicians. Let’s go over the main issues:
- Circulus in Probando (Circular Reasoning): The basic argument here is that since our quickie 361 tissue registration didn’t go through FDA approval and we are a legit 361, then it makes sense we have no FDA approval. That’s like saying a building and site review and approval is required before you build a house, but instead, you got a permit to build a fence that was issued online without a review, so you’re fine. That is until the city posts a “Stop-work” order on your front door.
- Homologous Schmologous. The document completely botches the idea of “homologous use” claiming that the company is fine with its quickie tissue registration since it only claims that its products can be used in a homologous way. That would mean that the company promotes using exosomes in the same way in which they are used in the human body. However, since the company can’t even tell which of the thousands of types of exosomes are in this product nor even if these exosome messages are consistent from vial to vial or still viable and functional, how could these exosomes be used in the same way they would be used in the body? For example, if they were capturing exosomes from growing placental cells, those messages are about growing a baby. Therefore homologous use would be growing a baby.
- Driving a truck through the eye of a needle. My favorite comment from the document is that the company has seized on one phrase from the FDA Warning which is “As a general matter…”. They claim that the FDA is using this as a special exemption that Direct Biologics somehow qualifies for, despite there being nothing from the FDA about this mystery exemption.
The Big Reasons this is a Drug
- As above, simply the claim of selling exosomes derived from someone else tissues (allogeneic) and the treatment claims on the website are enough to classify the product as a drug. Hard stop, do not pass Go.
- The fact that Direct Biologics is selling exosomes made from culture-expanded mesenchymal stem cells is by definition, more than minimal manipulation. Why? Culturing cells is more than minimal manipulation, hence any product made that way is a drug requiring full FDA clinical trials.
Kimera
In my opinion, another company playing this smokescreen game with the FDA public warning is Kimera. They have been selling exosomes for a few years and have not been shy about promoting all sorts of uses for their products. In fact, you can find videos where the company principals are claiming miraculous results in treating all sorts of incurable or lethal diseases.
If you want to see Kimera’s approach to the public warning, look no further than the video from the A4M conference which came out just one week after the warning. The argument that Kimera puts forth starts with the concept of a guidance statement that FDA issued in 2017, just after the Kimera lab got inspected for its amniotic fluid product:
So what did this say? That secreted body fluids like amniotic fluid are generally not considered HCT/Ps. Duncan Ross, Ph.D., the company founder, in the video I screenshotted above, makes the argument that since exosomes exist in amniotic fluid, exosomes can’t be classified as a 351 HCT/P drug. That, of course, is 100% opposite of what the warning says:

Selling Amniotic Fluid
You can bottle and sell amniotic fluid as a whole tissue under a quickie 361 tissue registration. You can filter the debris out via centrifugation and freeze it and still be within those 361 guidelines. However, you can’t make any claims about it other than how the body uses it, which would be for cushioning and support. The moment you start selling it as an “Exosome Product” and attaching treatment claims to it is when it’s no longer classified as a tissue but as a drug.
Playing Games
Dr. Ross above takes our amniotic fluid example much further. He states that since amniotic fluid is excreted by the body and has certain things in it that aren’t cells, then if he manufacturers those things, they aren’t regulated the same as drugs. Let’s dig in.
Let’s take Duncan’s concept further. We know that there are specific growth factors and other chemicals in amniotic fluid. In fact, it’s rich in hyaluronic acid (HA). This isn’t an HCT/P. So can I then manufacture HA using cells and avoid drug regulation?
We have companies that do this every day, but regrettably what they manufacture is a drug. Let’s use that HA example, which is an FDA regulated drug product. Traditionally HA was extracted from rooster combs, and now it is mainly produced via streptococcal fermentation. When companies removed it from rooster combs and tried to sell it as an injection to treat knee arthritis, it was a drug. Now that they make it in bioreactor vats, it’s still a drug. Duncan makes his exosomes by growing human cells in culture, which as discussed above is more than minimal manipulation, hence a drug product.
The Kimera 2017 FDA Inspection
Duncan also makes a big deal about an inspection report for his amniotic fluid product to treat knee arthritis from October of 2017, which of course is not the product that he makes by growing stem cells in culture and promotes to treat a host of incurable diseases. In addition, that inspection as listed on the report was a “limited inspection of this registered human tissue establishment”. So this discussion is a classic bait and switch.
Treatment Claims
Are there treatment claims? This is on Kimera medical director Doug Speil’s Facebook page:
Yes, these are treatment claims. Kimera exosomes are promoted to treat autoimmune diseases, musculoskeletal disorders, and nervous system disorders. Not only do we have treatment claims, but we also have a “How To” workshop for doctors on methods they should use to treat these diseases with Kimera exosomes!
Confusing Doctors 101
This work of exposing companies who are placing patients at risk and confusing physicians is time-consuming. This post took the better part of a full day to research and write and another morning to edit. As I have said before, it takes only minutes to spin a fabrication, but many hours to debunk it.
Physicians are confused as heck about exosomes. We have no clinical data that these products work. We have even less basic science data on how to make or deliver a consistent exosome product focused on tissue repair or reducing inflammation. The FDA tried to get ahead of this train wreck by issuing the public warning to consumers and physicians, but in no time the companies that in my opinion are clearly breaking the law were able to spin credible sounding arguments. However, once you look at the arguments in detail, they don’t hold water.
My bigger concern here is that it’s exactly this type of activity that is hurting the legit orthobiologics community. Because of companies like Direct Biologics and Kimera, we now have a huge wave of doctors who believe that they can use these products and advertise them as miracle cures. We have even more patients who have bought that hook, line, and sinker. Hence rather than taming the stem cell and exosome wild west, these companies create more havoc and a huge target for regulators to shut it all down-the legit players and the bad actors.
The upshot? This is frankly exhausting! The nutty arguments hatched by these two companies are not only keeping sales going, but they also convincing a whole host of gullible doctors to place themselves at risk by promoting unapproved drug products. Like I always say, you just can’t make this stuff up!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.