Dr. Singer Will Teach You How to Become a Stem Cell Millionaire
You’re a chiropractor whose business is built on $39 adjustments and then someone offers you a proposition. If you add “stem cell injections” you can treat everything from knees to aging and make millions in the process. All you need to do is to take a course and you’ll get everything you need to become a stem cell millionaire. Sounds far fetched? Regrettably, it’s happening every day. Let me explain.
Chiropractic Business Consultants
Physicians take for granted that we can be instantly jacked into insurance and hospital-based networks. Hence, few physician practices fail. Not so for chiropractic practices which have a high failure rate. Hence, business consultants in chiropractic are common.
One of the more popular chiropractic business consultants is David Singer. He usually teaches chiropractors how to pre-sell packages of adjustments, add ancillary services like physical therapy, or add a mid-level practitioner to prescribe hormones. However, many months ago he came on my radar when several of my local chiropractic colleagues reached out about the emails sent by David Singer pushing stem cells.
Becoming a Stem Cell Millionaire
To learn more, I signed up for one of David Singer’s free webinars. Regrettably, I had patients during that time slot, so I missed it. However, I got sent an automated email with marketing collateral for the stem cell program. Let’s just say that I was concerned.
This is from one of the PDFs sent to me:
“Knowing how to handle an audience is a science and an art. You will learn all the mechanics from your introduction to the close at the end. You will learn the secrets to signing up 100% of an audience as well as many simple procedures that have helped doctors to get to $300,000 a month with this material.”
Yikes! Dr. Singer tells you that if you add stem cells to your practice, he can collect up to an extra 3.6 million a year!
This is Painful
I usually have many interesting moments when reading the things that chiropractic stem cell marketing experts write and Dr. Singer’s “Regenerative Cellular Treatment” pamphlet was no different. In this, he discusses the five products he’s selling “for homologous use”. He even provides a reference to what he considers homologous use:
“Homologous – similar in position, structure, and evolutionary origin but not necessarily in function.”
However, that’s not the definition that the FDA uses, which is:
“As defined in 21 CFR 1271.3(c), homologous use means the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor.”
This is a critical concept for the 5 products that Dr. Singer is selling which he calls Relev (amniotic fluid), Reviv (amniotic fluid and membrane), Renyte (Wharton’s Jelly), Restor (Wharton’s Jelly + MSCs), and Recharg (umbilical cord blood). Why? Homologous here in the FDA’s parlance means that these products would never be approved for use in orthopedics without an actual FDA drug approval which these products don’t have. Meaning Wharton’s Jelly from an umbilical cord has little to do with muscles, tendons, and joints.
Regulatory Roulette?
After some research on where Dr. Singer was sourcing his products, I found out that these are the names for products that are actually sold by “New Life Regenerative Medicine”, about an hour from Dr. Singer’s Florida office. Dr. Singer’s materials state that Restor has mesenchymal stem cells (MSCs). The NewLife website reports that they’re “Medicinal Signaling Cells” (a newer term for MSCs). Why is that significant? That’s a clearly illegal claim.
How can I say that Dr. Singer’s and New Life’s claims are not legal? There have been many Warning Letters issued to companies with quickie 361 tissue registrations such as the one New Life filed which claim to be selling stem cells. Here’s one such letter to another vendor that the FDA issued that accuses it of marketing an unapproved drug product. This is now one of a series of letters that makes it crystal clear that any of these companies that are still selling products that claim to have live stem cells are not compliant. This is the key statement from this most recent letter:
“In addition, your products fail to meet other criteria set forth in 21 CFR 1271.10(a). The umbilical cord blood product, StemL UCB-Plus™, fails to meet 21 CFR 1271.10(a)(4). This product, manufactured from donated umbilical cord blood, is dependent on the metabolic activity of living cells for its primary function and is not for autologous use, allogeneic use in a first-degree or second-degree blood relative, or reproductive use. The umbilical cord product, SternL UCT-Plus™, fails to meet the minimal manipulation criterion set forth in 21 CFR 1271.10(a)(l) and defined for structural tissue in 21 CFR 1271.3(f)(1). The product does not meet this criterion because your processing alters the original relevant characteristics of the umbilical cord related to its utility for reconstruction, repair, or replacement.”
While that’s some dense regulatory speak, here’s what it all means:
- You claim that you’re selling live cells, which makes your product a drug that requires clinical trials and actual FDA approval-you never did that
- Your processing of the tissue alters what it was meant to do in the body, hence that’s another reason your product is a drug
What is Restor?
In fact, as I quickly learned, NewLife doesn’t make these products, as they are listed on the FDA TIR query as only a distributor. I called their President/CEO, Viki Mansavage, a 35-year veteran of the tissue banking industry. She discussed that their Restor product is manufactured by a third party. I also asked her why her website claims that there are “medicinal signaling cells” in their products, clearly, a claim that FDA says is not permitted for a 361 registered tissue product. She stated that she is trying to do the right thing and pursue an RMAT designation (a type of FDA approval), but when confronted with the concept that she is not permitted to make a claim that she is selling live cells until the FDA actually approves the results of clinical trials (which her company has not conducted), I got no clear response. Later, to Viki’s credit, New Life changed its website to get rid of the claim that the Restor product has MSCs.
So does Restor have MSCs and are there any tests that would meet ISCT guidelines that show that? No, Viki also admitted that it was very unlikely that this product has many live and functional stem cells. This statement goes in the opposite direction of what David Singers states in his marketing collateral. Let’s dig into Singer’s claims.
Umbilical Cord vs Bone Marrow Stem Cells
I again reviewed David Singer’s brochure entitled”Stem Cell Education”. Here he tells us:
“As we age, and our bodies slow down so do our stem cells…It starts out slow day one, 1 penny, day two, 2 pennies, day three, 4 pennies and so on and so on. By the end of the month its going from $200,000.00 one day $400,000.00 the next day and $800,000.00 the next day to $1,600,000.00 and so on. These cells do the same thing. A cell that has a doubling time of 20 to 24 (young sources cells) hours becomes 1,000,000,000 (that is billion) cells in 30 days. A cell that has a doubling time of 60 hours (someone 65 years of age) becomes 200 cells in 30 days. To help the body grow, fix, replace, and regenerate we need to have young vibrant healthy cells or “Day 1” cells meaning the cells are only one day old (newborn). Day one cells are considered young healthy robust cells that have the power to really make a difference in helping a patient’s body to heal.”
This is the standard chiro song and dance that many patients have bought into, that their cells are just too old and that there are millions of young stem cells in umbilical cord products that once injected, these cells will grow in many billions more in their bodies. Is any of this demonstrably true? Is there any evidence that Restor or any other umbilical cord product on the market has millions of live and viable MSCs that are rapidly growing?
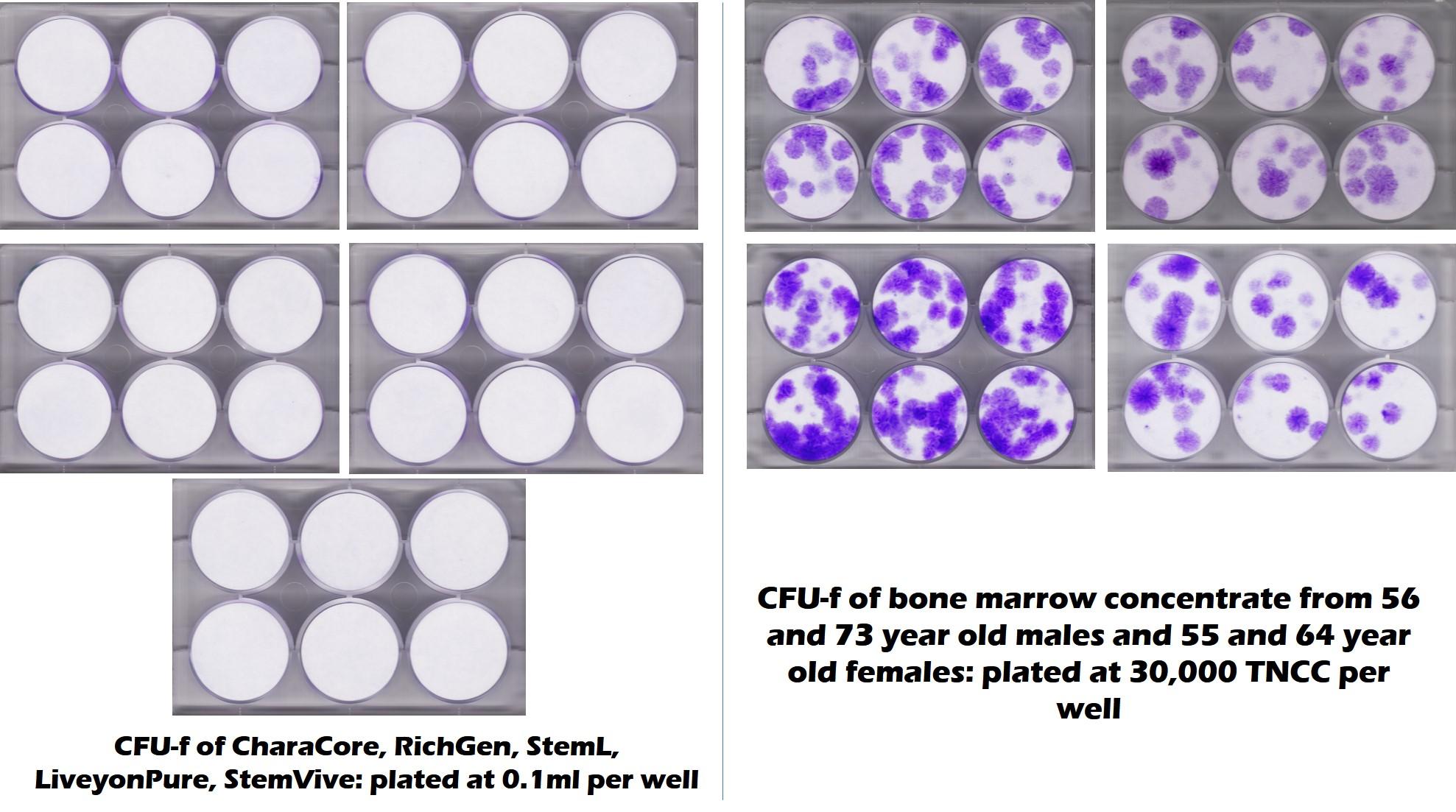
As I have written many times before, there is now quite a bit of research that the amniotic fluid and umbilical cord products that are being sold have no viable and functional mesenchymal stem cells MSCs) (1-3). In addition, this recent test of 5 commonly used umbilical cord products from the CSU Translational Medicine Institute tells the whole story visually. In this CFU-f test (one of the ISCT requirements for determining if you have MSCs (4)), purple dots represent stem cell colonies. White is no stem cells. As you can see, all 5 commercially available products on the left had no viable stem cells, while middle-aged and elderly bone marrow had tons of stem cells. So much for Dr. Singer’s sales pitch. In fact, if it were even a little true and there were ANY fast-growing MSCs with 24 hour doubling times in any of these products, given that this is about a 10-day test, the plates here on the left would be covered in one big mass of purple instead of being sterile white.
Dr. Singer’s Responses
I sent the following questions to Dr. Singer’s office and called his staff to ensure I had the correct email address:
- Is Dr. Singer aware of recent FDA actions in the way of Warning letters and TRG TRIP determinations that claims of umbilical cord mesenchymal stem cells make a 361 registered tissue an adulterated, misbranded, and unapproved drug product which is illegal to sell, distribute, or advertise?
- Dr. Singer’s collateral that I was sent clearly positions the Restor product as containing mesenchymal stem cells…Does Dr. Singer have a comment on that information?
- What information that meets ISCT guidelines can Dr. Singer provide me that clearly shows that Restor has live and functional MSCs?
- If no such information is available, what is Dr. Singer’s comment to the statement that his collateral is not accurate and is in fact misleading?
- Can Dr. Singer provide me information from the FTC or FDA that supports his ability to make claims that he is selling stem cell products?
- Can I get Dr. Singer’s publications, textbooks, or other published information that demonstrates that he is an expert in regenerative medicine?
At the time of this writing, I had no response. I will update this post if that changes.
Let’s Review
- We have a chiropractic business consultant with no expertise in regenerative medicine (i.e. no publications, book chapters, or anything else that defines expertise in medicine) who claims to be able to make chiropractors stem cell millionaires, with collections of up to 3.6 million a year.
- Dr. Singer’s collateral contains obvious errors in its understanding of simple terms like “homologous use”.
- His information claims that he is selling a product (Restor) with mesenchymal stem cells. However, this product is actually a brand that is a private relabelling of a different Wharton’s Jelly product. There is no clinical research on the rebranded product.
- The CEO of the company that rebrands the private labeled Restor product admits that it’s very unlikely that the product has any live and viable stem cells.
- Despite this Dr. Singer claims that it has “day 1 cells” that will rapidly proliferate versus the old and slow-growing cells in your bone marrow. None of the published data supports these claims, in fact, the opposite conclusion is supported.
The upshot? If you ever wondered why chiropractors claiming to deliver patients stem cell products but who are really delivering to them dead umbilical cord tissue are popping up all over the place, look no further than chiropractic business consultants like Dr. Singer. If regulators want to stop this stuff, they need to look at the whole food chain. Here we have a tissue manufacturer who supplies a distributor who is supplying chiropractors with a Wharton’s Jelly product that they claim has so many active cells with 20-24 hour doubling times that these will grow into billions of stem cells in your body. However, even the distributor admits that what she sells is stem cell poor, which fits with the research that shows that none of these products have any live and functional mesenchymal stem cells. You can’t make this stuff up.
_____________________________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. https://www.ncbi.nlm.nih.gov/pubmed/16923606

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
