The Great Disc Slide Caper: East West Health Review
[August 14th, 2020 update: East West Health received an FDA letter about its claims of using umbilical cord “stem cells” on Aug 11th, see this link.]
I have been chasing my disc slides around the Internet all year. They are being used by various fake stem cell clinics as a marketing tool, even though these clinics can’t replicate what was done to produce those results. That brings us to a review of East West Health, an acupuncture clinic that purports to offer stem cells.
Who is East West Health?
East Weat Health is an acupuncture and alternative medicine clinic in multiple locations including St George, Park City, and Salt Lake Utah as well as Albequerque New Mexico. The clinic claims to offer amniotic and umbilical cord stem cell injections and even has a book to download on the topic. So is any of this true? Does the clinic really offer stem cell injections?
My Disc Slides
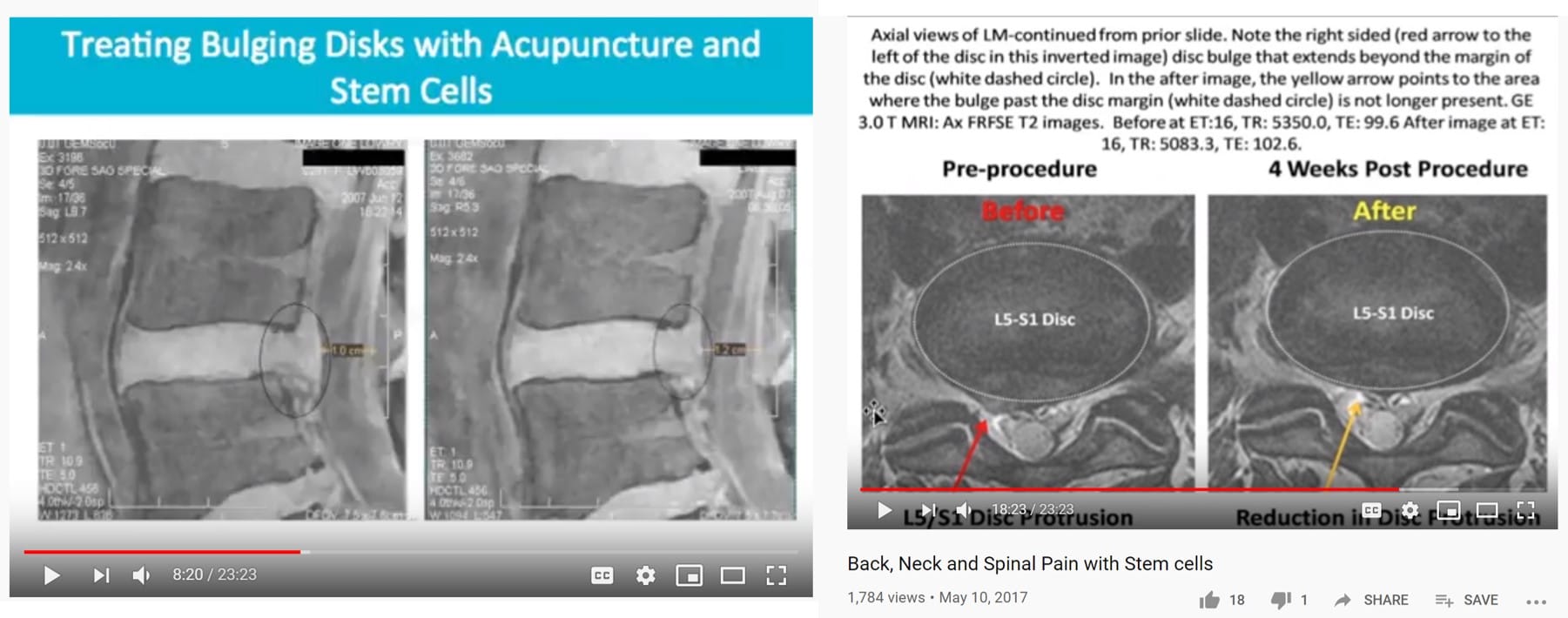
East West Health came on my radar recently when I ran across their YouTube video that had stolen my slides showing the before and after results of my patients with disc bulges (below). These slides show how our disc procedure was able to reduce the size of disc bulges after a precise, image-guided stem cell injection. The results are pretty amazing, which is why I turned these patient images into slides.
Given that I had not provided my permission for anyone to use these slides, I first checked into whether East West Health was even using the same procedure that produced those results. Why? Without using the same procedure, in my opinion, advertising the results would be fraud, so let’s dig in.
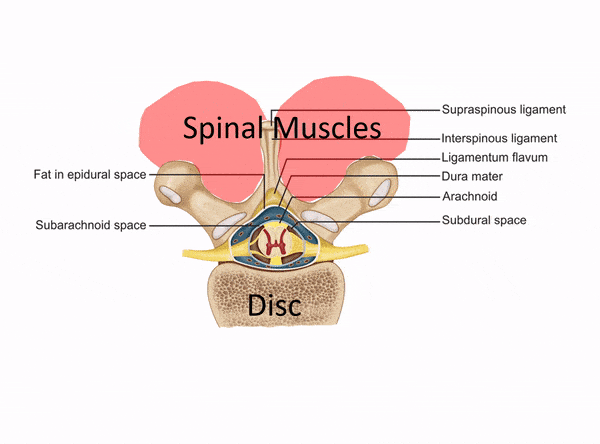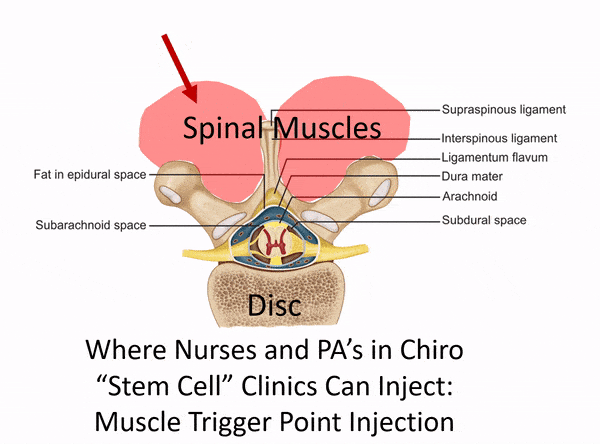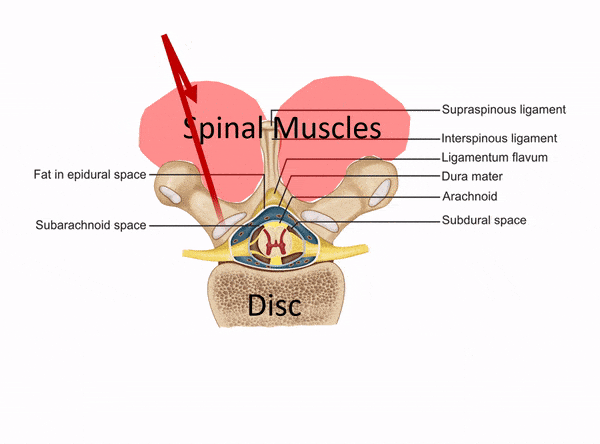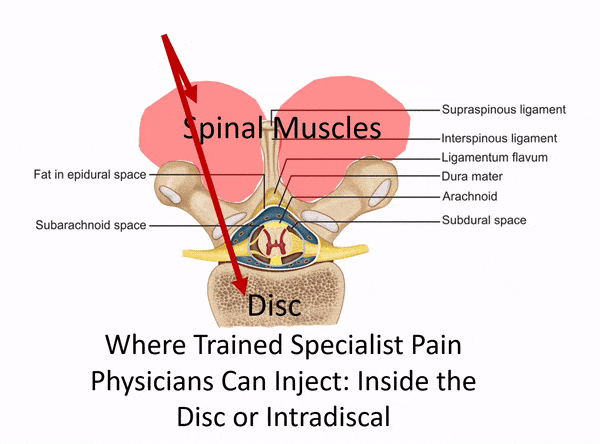
The Difference Between a Disc Injection and a Muscle Trigger Point Injection
The procedure above used an injection into the disc itself, which needs to be performed using c-arm fluoroscopy (real-time x-ray). This is a deep spinal injection that can only be safely performed by a medical super-specialist who has that specific training. East West Health only has two physicians among many acupuncturists. Neither of these doctors would have the training and expertise to be able to perform this type of intra-discal injection. In fact, they would only have the training to perform a muscle trigger point or superficial ligament injection. The difference is below:
What’s Injected…
The stem cell disc procedure seen above used some very special stem cells. These were first derived from the patient’s own bone marrow and then grown in culture for several weeks. In addition, the cells were conditioned using hypoxia to allow them to survive in the oxygen-poor disc environment.
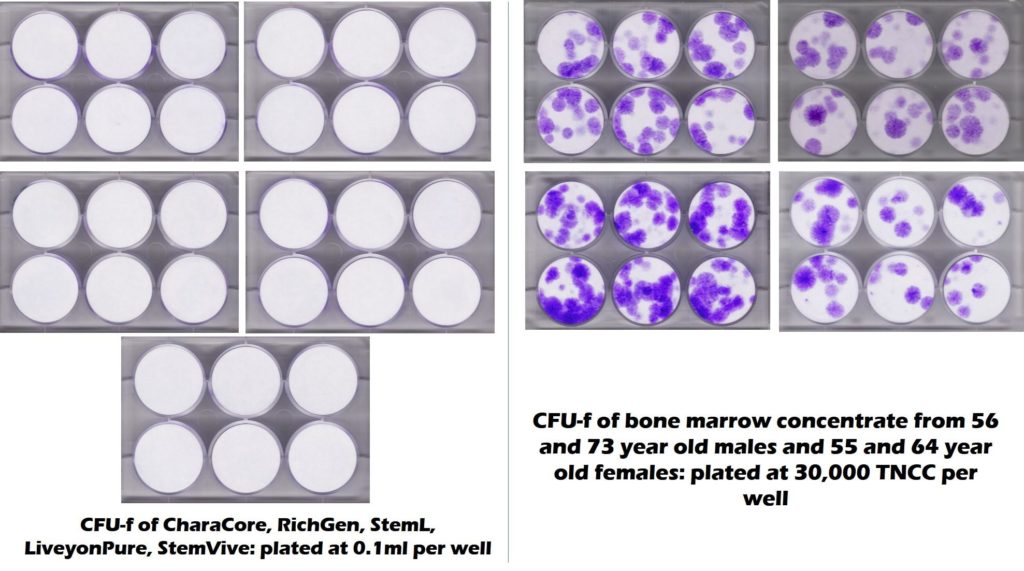
What does East West Health inject? They use amniotic and umbilical cord products produced by a nearby company called Utah Cord Bank. The CSU Translational Medicine Institute tested one of their Wharton’s jelly products called StemVive. The manufacturer claims that StemVive has live and functional mesenchymal stem cells. However, as you can see from the test results below, the 5 umbilical cord products on the left (including StemVive) didn’t have live and functional mesenchymal stem cells. In this test, those would show up as purple dots and all you see is sterile white. What does have purple dots on the right? Middle-aged and elderly bone marrow which does contain live and functional mesenchymal stem cells.
Hence, I injected bone marrow stem cells into those patient discs using precise x-ray guidance and what was injected by East West Health without x-ray guidance doesn’t have stem cells. So we’re talking apples and oranges here.
Digging Deeper
We also see this statement on the East West Health site:
“The umbilical cord stem cell injections used in treating patients with joint problems contain naturally developing anti-inflammatory agents that actually stimulates tissue repair. There have been no cases of patient rejection recorded so far. Over 10,000 injections have been used on patients without any cases of adverse side effects.”
Can this statement be verified anywhere? We know that 12 patients recently ended up in the ICU as a result of a contaminated umbilical cord “stem cell” product. We also know that umbilical cord blood needs to be matched with the patient or there can be serious side effects including organ failure and reported deaths (4-8). So where does this 10,000 number come from? I was unable to find a single published long-term safety study using the products from Utah Cord bank or any other 361 registered umbilical cord tissue manufacturer.
Is this Fraud?
I have dealt with a number of chiropractic and alternative health clinics this past few months. They all make the same claims about using amniotic and/or umbilical cord products that have stem cells, but research from multiple universities doesn’t back them up (1-3). They also all look to their tissue suppliers when I confront them about this problem and East West was no different. Instead of owning up to their false claim that they could produce similar disc results when they were incapable of replicating the procedure that was able to get those results or instead of providing data about their umbilical cord product that would meet ISCT guidelines, they asked that I come on their podcast to discuss this with their tissue supplier. I have seen the same behavior now many times, with many clinics taking the position that they are only parroting what is told to them by the company that sold them the umbilical cord tissue.
The problem with the concept that East West Health or XYZ chiro clinic is not responsible for these claims is of course ridiculous. The medical provider has a license and the public depends on them for accurate information. In my opinion, when they betray that trust by not doing their homework or getting educated enough to understand the basic science at a level required to challenge sales reps, the medical providers are liable.
The upshot? East West Health was and is advertising online things that are demonstrably untrue or can’t be verified. In my opinion, this is a violation of the public’s trust. Also in my opinion, they need to come clean with their patients that what they have claimed they offer is not what they are really offering.
_______________________________________
I reached out to East West Health on 11/26/19 and they emailed back stating that they would remove the slides from their presentation. However, at the time of this writing, I have not seen them remove the claims concerning the use of birth tissue stem cells in their clinics.
_______________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Eapen, Mary et al. Mismatched Related and Unrelated Donors for Allogeneic Hematopoietic Cell Transplantation for Adults with Hematologic Malignancies. Biology of Blood and Marrow Transplantation, Volume 20, Issue 10, 1485 – 1492. https://www.ncbi.nlm.nih.gov/pubmed/24862638
(5) Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. https://www.ncbi.nlm.nih.gov/pubmed/15191952
(6) Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood Jul 2014, 124 (3) 363-373; DOI: 10.1182/blood-2014-01-514786
(7) Lee SJ. Classification systems for chronic graft-versus-host disease. Blood Jan 2017, 129 (1) 30-37; DOI: 10.1182/blood-2016-07-686642
(8) Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood Jul 2014, 124 (3) 374-384; DOI: 10.1182/blood-2014-01-514752

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.