GoodCell Biobanking? An Insurance Policy or Something Else?

Credit: Shutterstock
As you know, I tend to write about what I experience daily. In my news feed this week, an ad popped up for “Good Cells,” and clicking through, I discovered that this was a blood-based biobanking service. Hence, I thought I would review the concept and some of the claims while educating about stem cells in your blood. Let’s dig in.
What is Biobanking?
I’ve been involved with storing culture-expanded, bone marrow derived, mesenchymal stem cells since 2005. That’s a type of biobanking, and the way that works is that the cells are isolated, culture-expanded, and then cryopreserved in liquid nitrogen. They can last almost indefinitely and be taken out when needed for the patient. Getting cells out for treatment takes about three days in recovery culture to get the cells healthy. This all takes place in Grand Cayman, where the use of autologous culture-expanded cells is considered the practice of medicine and not a prescription drug.
My Past Blogs on Companies Trying to Offer Consumer Cell Biobanking
While banking umbilical cords from newborns has become a big business here in the US, few companies have been successful in the adult cell biobanking business. Here are my past blogs on the companies that have tried to make a go of this:
All of these services had a fatal flaw. However, I’m open to the idea that GoodCell is different, so let’s dig into what they’re advertising.
GoodCell
When I clicked through on the ad above, this is what greeted me:
GoodCell Website
So we know that we need to be ready for the age of cell therapy to treat our diseases. Let’s find out more.

Credit: GoodCell Website
So GoodCell here is using the number of clinical trials underway in cell therapy of all types to get us to buy this product. Let’s dive deeper here:

Credit: GoodCell Website
These are interesting claims. Basically, as the FDA approves various cells for use, this will allow you to use your blood-based biobank for treatment.
How Does this Work?
From reading further into the website, it looks like you go to a Quest lab and have your blood drawn, and they ship it to GoodCell. What GoodCell does to your blood is less clear on the website, but the idea of IPS cells is brought up. That seems to mean that in the future, they intend to create heavily modified cells from your blood and then culture expand those for your treatment.
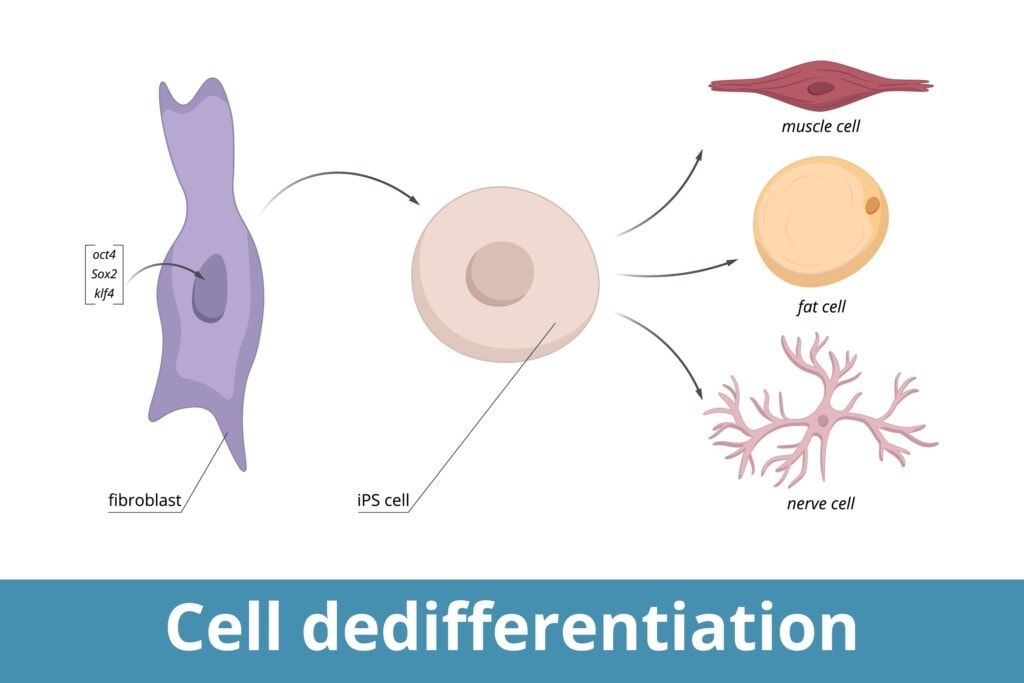
Credit: Shutterstock
What are IPS Cells? The idea that you can take normal human cells and dedifferentiate them back to their stem cell state has been known for almost two decades and is called induced pluripotency (14). The biggest problem is that all of the acquired mutations of those cells, like the ones from UV damage, get passed into the IPS cell (15). Meaning that there is no biologic free lunch. If you take old cells with lots of mutations and turn them into stem cells with IPS you get old stem cells with lots of mutations. Hence, it’s unknown why you wouldn’t just take your stem cells when you get sick and use them, especially if you’re older when you pull the trigger on GoodCell.
Let’s now look into two practical realities that make this biobanking play impractical at best and an insurance policy that may never pay off at worst.
What Type of Stem Cells are in Peripheral Blood?
While GoodCell seems to have plans to create IPS cells, let’s use this as an opportunity to review what kind of stem cells natively live in your blood. After that, we’ll explore regulatory hurdles to this kind of biobanking play.
Many years ago, we began working with a company that offered apheresis of peripheral blood and advertised that patients could bank this and use it in the future. This was a much more intense regimen than that used by GoodCell. It involved the patient taking a stem cell mobilizing drug that could push these cells from the bone marrow into the blood and not just a simple venous blood collection but the use of an apheresis machine. This machine pulled out many, many more peripheral blood nucleated cells than the process used by GoodCell. Despite that, in multiple tries with several samples, even though we were very experienced in growing mesenchymal stem cells from bone marrow, we could never get any adherent MSCs to grow from one of these more intensive collections. Why?
The kinds of stem cells that could be in the peripheral blood in normal daily circulations would include:
- Mesenchymal Stem Cells (MSCs)
- Hematopoietic Stem Cells (HSCs)
- Very Small Embryonic Like (VSEL) Cells
- MUSE Cells
The reason that we couldn’t get cells to grow from these collections is revealed below. Basically, these cells occur in very low numbers in your blood.
Mesenchymal Stem Cells
If you had to get one type of stem cell from peripheral blood, the all-star you would want would be MSCs. Normally MSCs only live in the bone marrow, fat, and other specific tissues with the MSCs that reside in the bone marrow being called Bone Marrow-MSCs (BM-MSCs). However, you can give the patient special mobilizing drugs which will push the BM-MSCs out of the bone marrow and into the peripheral blood (12). However, do MSCs live in the peripheral blood without mobilization?
Another MSC type called peripheral blood-derived MSCs (PB-MSCs) has been researched. Usually, these exist at a very low level in healthy individuals, but in some serious diseases, the number of circulating MSCs increases significantly (13). We’ll see that same trend over and over below. Stem cells show up in your blood in greater numbers when the proverbial $h%t hits the fan.
MUSE Cells
MUSE cells are fascinating because they are constantly mobilized from the peripheral blood to every organ and can replace damaged cells by differentiating into those cells (1,2). However, that tends to happen more often when serious problems are afoot, like cardiac arrest. MUSE stands for Multi-lineage differentiating Stress Enduring cell. So again, we see stem cells in the blood when there are serious problems with the patient.
Very Small Embryonic Like Cells (VSEL Cells)
VSELs were discovered by Ratajczak, and in humans, they are about the size of a red blood cell (5). It is thought that these cells originate in an embryo and live in every organ as the embryo grows. They also act as a backup system for tissue-committed stem cells. However, there is some controversy over whether they are real.
The basic concept for VSELs and the MUSE cells described above is that if the local stem cells in a specific area were depleted or couldn’t function, VSELs would step in to help (4,6,7,8). VSELs are usually in hibernation mode but are activated during stressful situations and are thought to give rise to mesenchymal stem cells.
VSELs are also very controversial. In 2013, one of the world’s experts in embryonic stem cells published a paper that blew up the VSEL research world (3). Basically, Dr. Weisman’s team tried to find VSELs in mouse bone marrow and didn’t find any.
In response to the 2013 study, Dr. Ratajczak later wrote:
“However, while we were working on better characterizing these cells and exploring possible applications in animal models in vivo, the very existence of VSELs was questioned. It is regrettable that, having a problem with VSEL purification, which requires a special gating protocol, this group did not follow the detailed protocol for VSEL isolation previously published in Current Cytometry Protocols (https://currentprotocols.onlinelibrary.wiley.com/doi/abs/10.1002/0471142956.cy0929s51). This paper slowed progress in this area.”
Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) make new blood cells and live primarily in the bone marrow, but they also can be found in low numbers in the peripheral blood (9,10). The good news is that HSCs can be used to replace muscle satellite cells (muscle stem cells) in cases where the patient is severely ill (11). Again, we see that same trend; stem cells mobilize into the bloodstream only in specific circumstances of serious disease.
Will This Insurance Policy Pay Off?
In many ways, any of these personal stem cell storage plays is like an insurance policy. Meaning you buy the product hoping that by saving these cells, they can be used to treat you in the future. However, is that possible?
First, as you can see above, the stem cells you would be interested in are all present in meager numbers in the peripheral blood of a normal healthy person. Hence, if we take a blood sample without giving the patient very specialized drugs, what are we collecting in the stem cell department? Not much.
However, GoodCells plans to overcome this by creating IPS cells. However, now we have a bigger problem called the FDA.
Regulatory 101 on Stem Cells
So let’s say you have your blood stored. Ten years from now, the FDA approves using IPS cells to treat knee arthritis. You then develop that condition, so you call up Good Cell or whatever company has your cells on ice, and then you make a withdrawal? Not so fast.
First, that approval you’re trying to piggyback off of was for allogeneic IPS cells, which is a very specific process of collecting cells from certain people and growing them in one specific lab. The FDA only approved that process with donor cells and NOT any of your cells stored with GoodCells. Hence, this approval will not allow you to use your cells.
The same scenario will be repeated time and time again. An FDA approval will come through and have nothing to do with your blood cells in the bank. In fact, the only way that GoodCells or any of these companies could remedy this situation is to get FDA approval for that specific cell source processed the way that this company does it. Given that this will cost 50-100 million USD a pop and a decade each, it’s very unlikely that any cell banking company could afford this kind of approval. In addition, each approval only covers one clinical condition. Hence, how could any company afford to get dozens of FDA approvals?
An Insurance Policy that Won’t Pay Off
IMHO, GoodCells, like every personal cell banking play I have ever seen, has a problem. In this case, the number of stem cells in normal healthy peripheral blood is poor. If we can get past that by creating IPS cells, we have a significant FDA approval problem. Hence, I’m unsure how any of these cell insurance policies ever pay off.
The upshot? Given my understanding of the existing science and regulatory process, it’s uncertain how this blood collection and cryopreservation process makes sense. I would have loved a world where your cells collected for personal use could be used by you in the way you saw fit, but in the United States, that’s not how the regulatory regime has unfolded.
_____________________________________________________________________
References:
(1) Yamashita T, Kushida Y, Abe K, Dezawa M. Non-Tumorigenic Pluripotent Reparative Muse Cells Provide a New Therapeutic Approach for Neurologic Diseases. Cells. 2021;10(4):961. Published 2021 Apr 20. doi:10.3390/cells10040961
(2) Kushida Y, Wakao S, Dezawa M. Muse Cells Are Endogenous Reparative Stem Cells. Adv Exp Med Biol. 2018;1103:43-68. doi: 10.1007/978-4-431-56847-6_3. PMID: 30484223.
(3) Miyanishi M, Mori Y, Seita J, Chen JY, Karten S, Chan CK, Nakauchi H, Weissman IL. Do pluripotent stem cells exist in adult mice as very small embryonic stem cells? Stem Cell Reports. 2013 Jul 24;1(2):198-208. doi: 10.1016/j.stemcr.2013.07.001. PMID: 24052953; PMCID: PMC3757755.
(4) Bhartiya D, Shaikh A, Anand S, Patel H, Kapoor S, Sriraman K, Parte S, Unni S. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod Update. 2016 Dec;23(1):41-76. doi: 10.1093/humupd/dmw030. Epub 2016 Sep 10. PMID: 27614362.
(5) Wojakowski W, Ratajczak MZ, Tendera M. Mobilization of very small embryonic-like stem cells in acute coronary syndromes and stroke. Herz. 2010 Oct;35(7):467-72. doi: 10.1007/s00059-010-3389-0. PMID: 20981396.
(6) Guerin CL, Blandinières A, Planquette B, Silvestre JS, Israel-Biet D, Sanchez O, Smadja DM. Very Small Embryonic-like Stem Cells Are Mobilized in Human Peripheral Blood during Hypoxemic COPD Exacerbations and Pulmonary Hypertension. Stem Cell Rev Rep. 2017 Aug;13(4):561-566. doi: 10.1007/s12015-017-9732-6. PMID: 28285391.
(7) Gharib SA, Dayyat EA, Khalyfa A, Kim J, Clair HB, Kucia M, Gozal D. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic-like stem cells and activates developmental transcriptional programs in mice. Sleep. 2010 Nov;33(11):1439-46. doi: 10.1093/sleep/33.11.1439. PMID: 21102985; PMCID: PMC2954693.
(8) Skirecki T, Mikaszewska-Sokolewicz M, Godlewska M, Dołęgowska B, Czubak J, Hoser G, Kawiak J, Zielińska-Borkowska U. Mobilization of Stem and Progenitor Cells in Septic Shock Patients. Sci Rep. 2019 Mar 1;9(1):3289. doi: 10.1038/s41598-019-39772-4. PMID: 30824730; PMCID: PMC6397313.
(9) Amouzegar A, Dey BR, Spitzer TR. Peripheral Blood or Bone Marrow Stem Cells? Practical Considerations in Hematopoietic Stem Cell Transplantation. Transfus Med Rev. 2019 Jan;33(1):43-50. doi: 10.1016/j.tmrv.2018.11.003. Epub 2018 Nov 12. PMID: 30528986.
(10) Jansen J, Hanks S, Thompson JM, Dugan MJ, Akard LP. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005 Jan-Mar;9(1):37-50. doi: 10.1111/j.1582-4934.2005.tb00335.x. PMID: 15784163; PMCID: PMC6741412.
(11) Asakura A. Skeletal Muscle-derived Hematopoietic Stem Cells: Muscular Dystrophy Therapy by Bone Marrow Transplantation. J Stem Cell Res Ther. 2012;Suppl 11:005. doi:10.4172/2157-7633.S11-005
(12) Villaron EM, Almeida J, López-Holgado N, Alcoceba M, Sánchez-Abarca LI, Sanchez-Guijo FM, Alberca M, Pérez-Simon JA, San Miguel JF, Del Cañizo MC. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004 Dec;89(12):1421-7. PMID: 15590390.
(13) Xu, Liangliang & Li, Gang. (2014). Circulating mesenchymal stem cells and their clinical implications. Journal of Orthopaedic Translation. 2. 1–7. 10.1016/j.jot.2013.11.002.
(14) Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663-76. doi: 10.1016/j.cell.2006.07.024. Epub 2006 Aug 10. PMID: 16904174.
(15) D’Antonio M, Benaglio P, Jakubosky D, et al. Insights into the Mutational Burden of Human Induced Pluripotent Stem Cells from an Integrative Multi-Omics Approach. Cell Rep. 2018;24(4):883-894. doi:10.1016/j.celrep.2018.06.091

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
