Interspinous Spacers: The Best Thing Since Sliced Bread or a Bad Idea?
Lumbar stenosis is a big problem that can make it hard to stand upright, so invasive surgery is often recommended. However, over the last five years, a new device category called an interspinous spacer has emerged that promises to solve this problem with minimally invasive surgery. However, is this a good idea? Let’s dig in.
Lumbar Stenosis
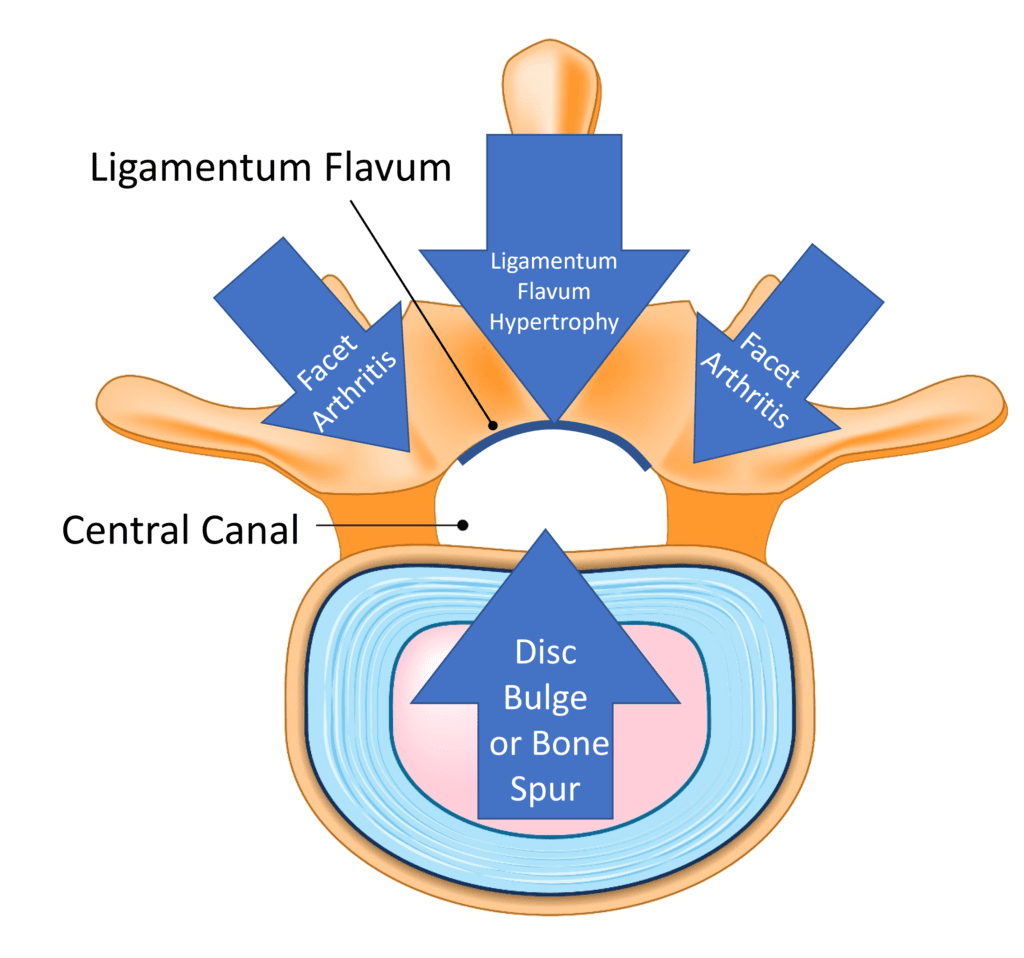
Credit: Shutterstock with Illustrations on Stock Image by Author
The basic idea behind spinal stenosis is that the place where the spinal nerves or cord travels through gets too small and irritates that nerve, causing symptoms. Lumbar stenosis comes in two flavors: central canal and foraminal. The former involves the main canal in the middle of the vertebrae where the spinal nerves go to and from the spinal cord and brain. This canal can have added pressure from the ligamentum flavum in the back, a disc bulge or bone spur in the front, or enlarged facet joints on either side.
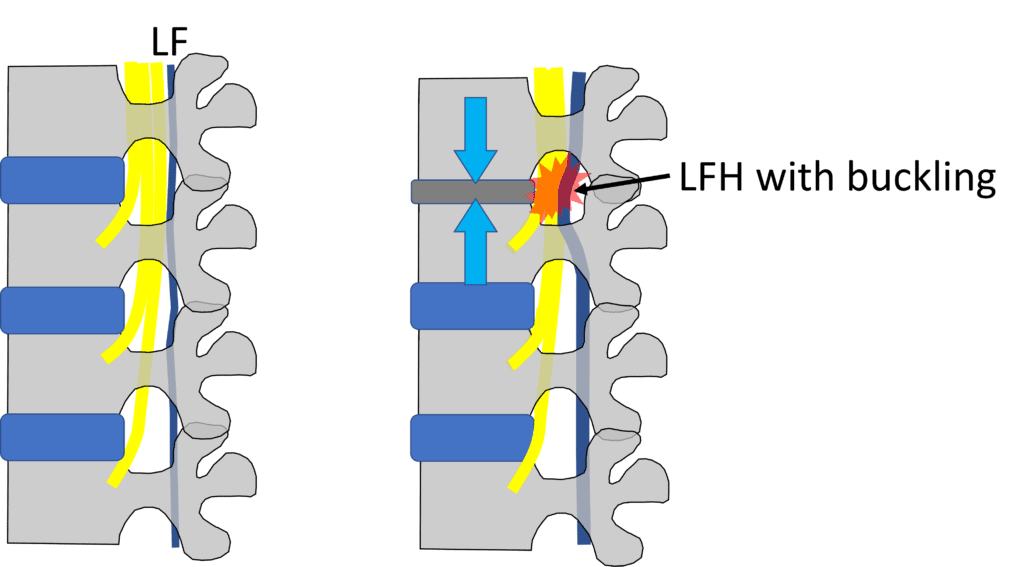
Credit: Illustration by Author
Above, I have focused on central canal stenosis involving ligamentum flavum (LF) hypertrophy (LFH) with buckling, which is the main therapeutic target of an interspinous spacer. The LF runs in the back of the spinal canal. When the spinal level gets degenerative and loses disc height (as shown in the top level on the right above), this structure can become hypertrophied (bigger) and buckle as the degenerative disc loses height. This can irritate the descending spinal nerves (shown by the red star). This bucking of the LF gets better with flexion (bending forward), as do symptoms, and gets worse with extension (standing straight or bending backward), which also worsens symptoms.
When central canal stenosis occurs, patients have difficulty standing up straight, which can cause severe low back pain and/or numbness or weakness in the legs. The most common treatment being used, beyond physical therapy, begins with interventional pain management, like epidural steroid injections or radiofrequency ablation (RFA). When this doesn’t work, the next step is usually a wide laminectomy (surgical decompression), cutting out the structures that irritate the nerves in the spinal canal and fusing this level. Given that this is a big surgery with many possible serious side effects, there has been a search for other options that don’t involve open surgery.
Interspinous Spacers 101
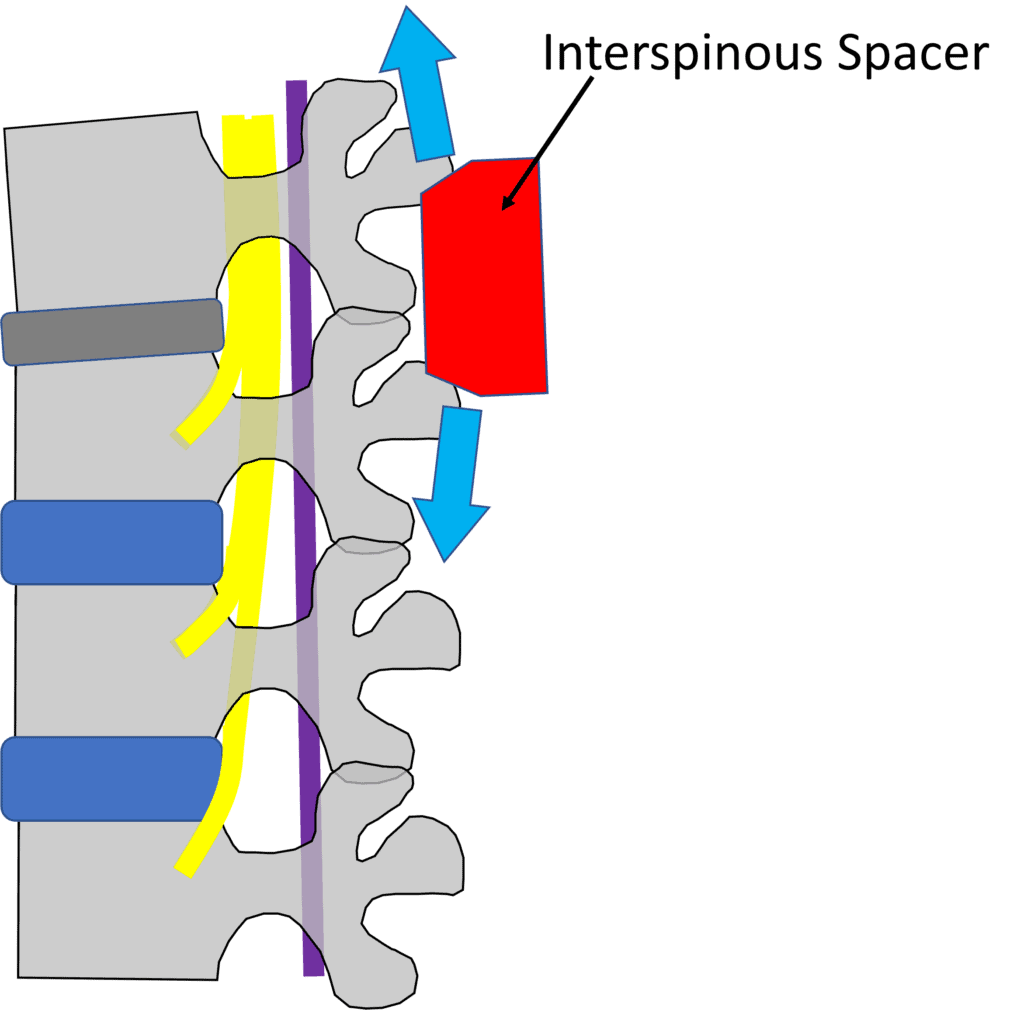
Credit: Illustration by Author
The idea behind an interspinous spacer is to create height at the back of the spinal column, pulling the buckled LF out of the spinal canal and opening up room for the spinal nerves. Given that the spacer can be inserted via a minimally invasive technique by surgeons and pain management doctors, what’s not to like?
These spacers go by the following brand names (by market share) (1):
- Globus Medical-Aerial, SP-FIX, RENEGADE, SP-FLEX
- Zimmer Biomet-Wallis SYSTEM, ASPEN, InterBRIDGE,
- Paradigm Spine-Coflex
- Meditronic-DIAM, X-Stop
- Life Spine-Aileron TRX
- Vertiflex
- Nuvasive-Affix II Mini
- Spine Frontier-Inspan
This is a selection of devices to show you what they look like:

The spacers above that have teeth are used more for posterior fusion, and the ones with smooth sides are used to open up the back of the spinal canal.
Do Insterspinous Spacers Work?
My search for research supporting that interspinous spacers are a great idea began looking for meta-analyses. These research papers summarize all the research for a given treatment. A 2014 analysis looked at two RCTs and three case series and found that while these devices could help back and leg symptoms, they were associated with a three times higher reoperation rate over traditional larger back surgery (decompression) (2). A 2016 meta-analysis found that while these devices had about half the complication rates compared to decompressive surgery, they had a three times greater reoperation rate (3). Another 2015 larger meta-analysis found that this entire surgical therapeutic space for low back stenosis has a POOR evidence base as none of these procedures (surgical decompression or interspinous spacers) have ever been compared against sham surgery (4). That’s a HUGE problem for an invasive procedure, as the placebo effect of big surgical procedures is massive (5). How big? More than half of the patients who receive a faked low back surgery that doesn’t decompress anything reports significant improvements from the sham procedure. The 2015 meta-analysis did report that interspinous spacers were slightly more effective than surgical decompression but had four times the reoperation rate.
Let’s look at another study by Schenck et al. because, at first glance, it looks great, but anybody who has spent time publishing research will realize the glaring problem (12). These authors looked at 159 patients who were randomized into an interspinous spacer or surgical decompression to treat central canal stenosis. These patients were followed for five years. The reoperation rate was, again, a ridiculously high 29% at two years. They then looked at the “success rate” for the interspinous device group at five years and found it was similar to surgery, but there’s a serious problem in making that comparison. Why? Because 29% of the patients who responded poorly were pulled out of the data set by getting another operation!
What are the Published Complications of Using an Interspinous Spacer?
Why are all of these reoperations happening? Remember that means that another surgical procedure is required to fix a problem created by the first surgery. Here I tried to stay away from company-funded studies and focus on independent surgeons reporting their results to colleagues. Let’s review.
Bowers et al. reported that they used the X-Stop device on 13 patients, with 9 of 13 having severe central canal stenosis, and then followed them up on average 43 months later (6). Initially, pain improved on average by 72%, but the pain returned in 77% of the patients by the time of the follow-up. The complication rate was VERY HIGH at 38%, which included three spinous process fractures and two new instances of spinal nerve root compression.
Another study that followed patients for less time at 23 months showed a serious complication rate of 12% (7). Other studies, funded mainly by the companies making these devices show fewer complications. This is a common problem when looking at the literature concerning surgical devices. Why? The business model.
The Surgical Device Business Model is a Problem
Why do company-funded studies often show no problems or few complications from a device, while independent publications show serious complications? The problem begins with the markup on these devices. Let me explain.
Look at the devices above in the context of what you can buy on Amazon right now. It’s not hard to find complex devices made of anodized aluminum or stainless steel selling for $20-40. If we include simple devices like the one shown on the far left above, you can get that down to $5-10. That means manufacturing costs in China or India are in the $3 to $20 range. However, unlike products on Amazon, the devices shown above sell for $4-8000 and up (10). That leaves gobs of money for profits. Where does that extra money go?
Take, for example, Zimmer-Biomet, which paid $109,000,000 to doctors in 2018, which is the problem in this entire space (11). It’s easy to find ways to get money to surgeons and pain management doctors who implant your devices.
What’s Not to Like?
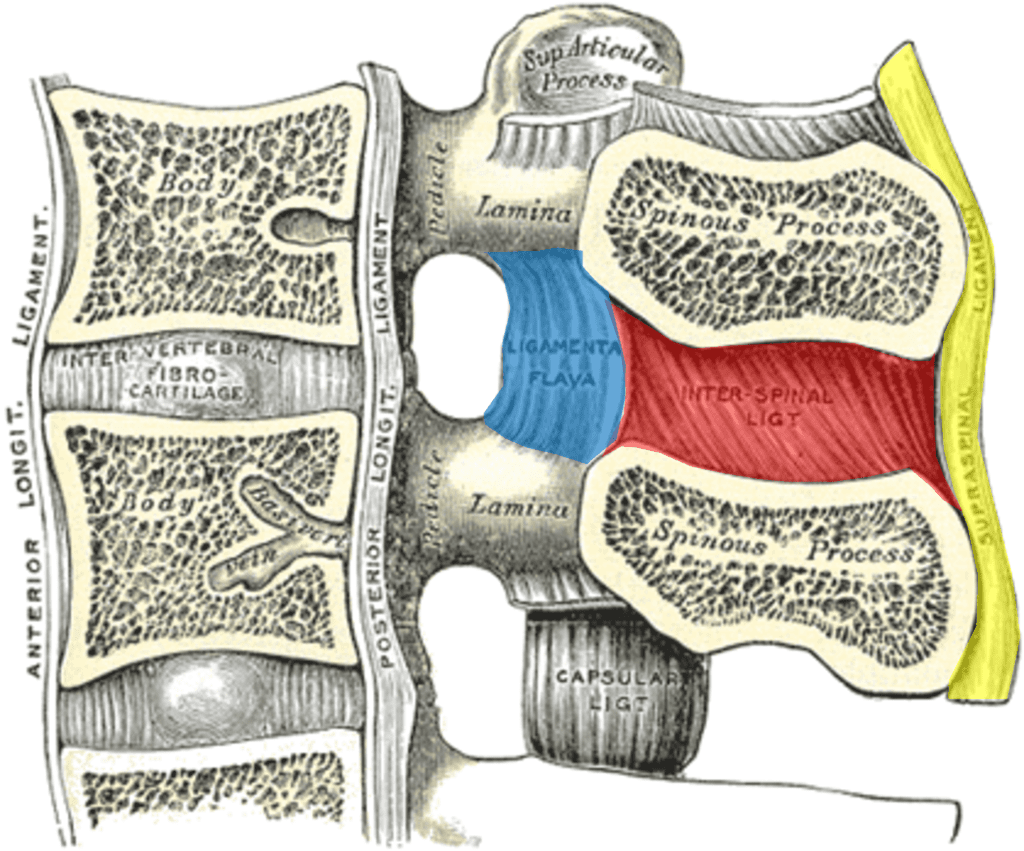
Credit: Wikipedia Commons with Edits by Author
Now let’s get into my comment, “What’s not to like?” To understand that, we need to dive back into anatomy.
You probably know that there is a disc at each level in the front of the spine, but there are also strong ligaments in the back. As shown above, in yellow, we see the supraspinous ligament and the interspinous ligament in red. Both of those connect to the ligamentum flavum, shown in blue. All of these form a suspension bridge structure, as shown below:
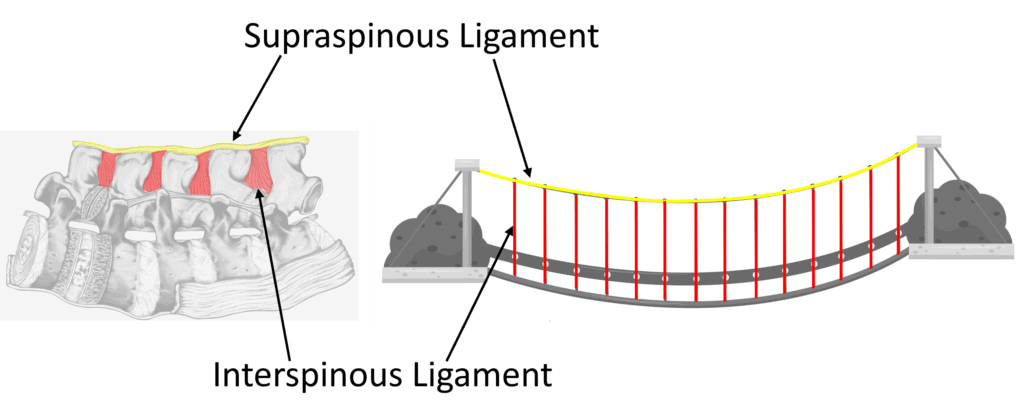
On the left above, I have turned the lumbar spine on its side, just like when you bend forward to pick something up. In that case, the yellow supraspinous and red interspinous ligaments act as a support system that keeps the vertebrae from falling forward. In this type of system, one link in the suspension bridge depends on the tension of the other to be efficient. If your spine flexes, these ligaments get tighter and help you lift something from the floor by taking much of that load.
So what do you think happens when a physician bangs an interspinous spacer where the supraspinous and interspinous ligaments are located? Those ligament links between the individual vertebra are forever damaged, and with it, the entire biomechanical system that was designed to handle loads when you bend.
There’s also another big obvious biomechanical problem with interspinous spacers:
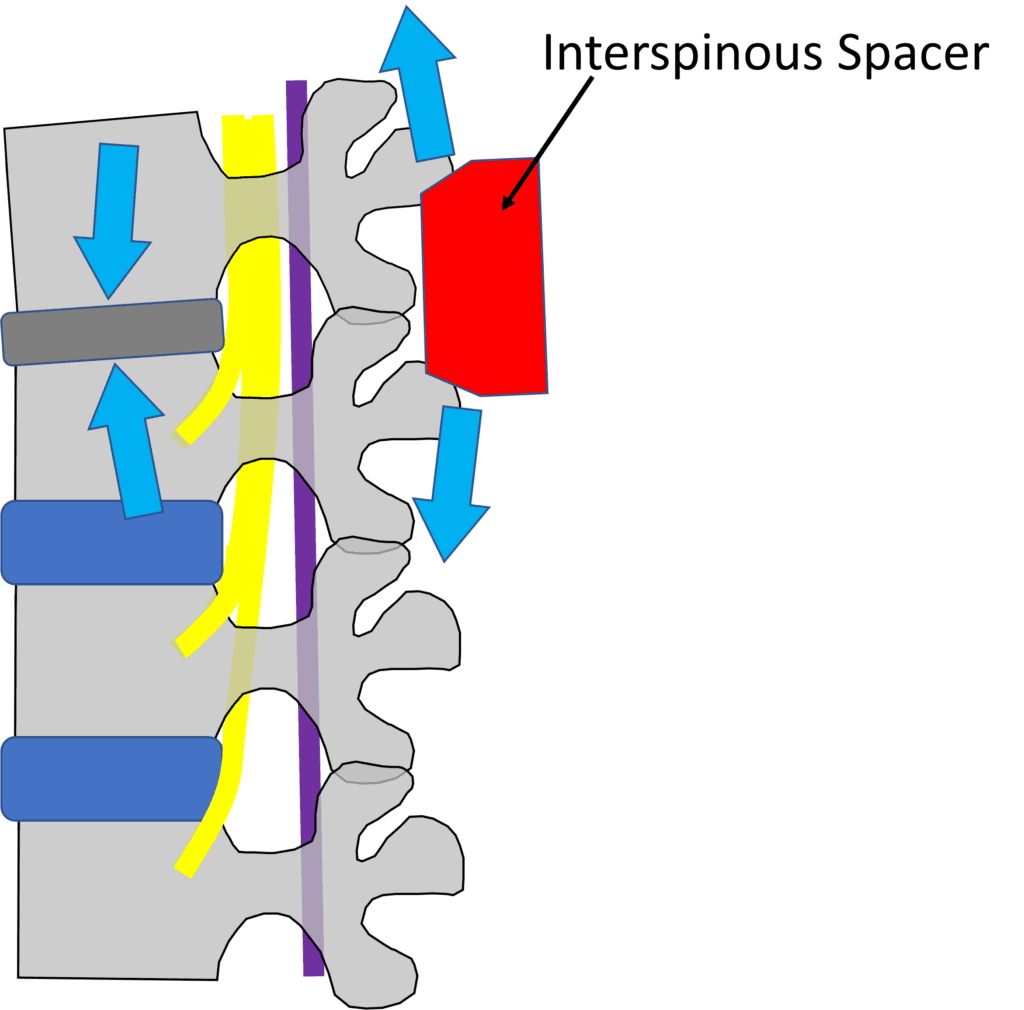
Illustrations by Author
Notice above that as you distract the spinous processes apart, that force goes onto the already degenerated disc! This virtually ensures more problems upfront. No wonder the reoperation rate for these devices is sky-high as you’re fixing one problem and creating another.
Is There a Better Way?
Why not treat the misbehaving ligaments rather than destroy them with surgery? That’s what we’ve been doing with targeted platelet-rich plasma injections for years. This involves treating the functional spinal unit, and this approach has been featured in two publications (8,9). When stenosis is involved, in my clinical experience, injection of the hypertrophied ligamentum flavum using precise x-ray guidance provides just enough room in the spinal canal as the patient moves to allow them to stand and walk.
The upshot? Interspinous spacers damage the normal biomechanics of the spine. The research shows that this treatment, on balance, is no better than old-fashioned low back surgery. It also permanently destroys critical parts of the spine.
____________________________________________________________________
References:
(1) Spine Market Group. 10 Interspinous Devices to Know…! https://thespinemarketgroup.com/10-interspinous-devices-to-know/ Accessed 12/18/22
(2) Wu AM, Zhou Y, Li QL, Wu XL, Jin YL, Luo P, Chi YL, Wang XY. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS One. 2014 May 8;9(5):e97142. doi: 10.1371/journal.pone.0097142. PMID: 24809680; PMCID: PMC4014612.
(3) Phan K, Rao PJ, Ball JR, Mobbs RJ. Interspinous process spacers versus traditional decompression for lumbar spinal stenosis: systematic review and meta-analysis. J Spine Surg. 2016 Mar;2(1):31-40. doi: 10.21037/jss.2016.01.07. PMID: 27683693; PMCID: PMC5039840.
(4) Machado GC, Ferreira PH, Harris IA, Pinheiro MB, Koes BW, van Tulder M, Rzewuska M, Maher CG, Ferreira ML. Effectiveness of surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS One. 2015 Mar 30;10(3):e0122800. doi: 10.1371/journal.pone.0122800. PMID: 25822730; PMCID: PMC4378944.
(5) Jamjoom AM, Saeedi RJ, Jamjoom AB. Placebo Effect of Sham Spine Procedures in Chronic Low Back Pain: A Systematic Review. J Pain Res. 2021;14:3057-3065
https://doi.org/10.2147/JPR.S317697
(6) Bowers, C., Amini, A., Dailey, A. T., & Schmidt, M. H. (2010). Dynamic interspinous process stabilization: review of complications associated with the X-Stop device, Neurosurgical Focus FOC, 28(6), E8. Retrieved Dec 18, 2022, from https://thejns.org/focus/view/journals/neurosurg-focus/28/6/2010.3.focus1047.xml
(7) Barbagallo GM, Olindo G, Corbino L, Albanese V. Analysis of complications in patients treated with the X-Stop Interspinous Process Decompression System: proposal for a novel anatomic scoring system for patient selection and review of the literature. Neurosurgery. 2009 Jul;65(1):111-19; discussion 119-20. doi: 10.1227/01.NEU.0000346254.07116.31. PMID: 19574832.
(8) Williams C, Jerome M, Fausel C, Dodson E, Stemper I, Centeno C. Regenerative Injection Treatments Utilizing Platelet Products and Prolotherapy for Cervical Spine Pain: A Functional Spinal Unit Approach. Cureus. 2021 Oct 8;13(10):e18608. doi: 10.7759/cureus.18608. PMID: 34659923; PMCID: PMC8500543.
(9) Atluri S, Murphy MB, Dragella R, Herrera J, Boachie-Adjei K, Bhati S, Manocha V, Boddu N, Yerramsetty P, Syed Z, Ganjam M, Jain D, Syed Z, Grandhi N, Manchikanti L. Evaluation of the Effectiveness of Autologous Bone Marrow Mesenchymal Stem Cells in the Treatment of Chronic Low Back Pain Due to Severe Lumbar Spinal Degeneration: A 12-Month, Open-Label, Prospective Controlled Trial. Pain Physician. 2022 Mar;25(2):193-207. PMID: 35322978.
(10) Auerbach et al. PSU10 Comparative Cost-Effectiveness Analysis of Coflex Interlaminar Stabilization Versus Posterolateral Fusion for Lumbar Stenosis and Low-Grade Spondylolisthesis. Value in Health. VOLUME 15, ISSUE 4, PA75, JUNE 01, 2012
(11) ProPublica. Dollars for Docs. https://projects.propublica.org/docdollars/company. Accessed 12/19/22.
(12) Schenck CD, Terpstra SES, Moojen WA, van Zwet E, Peul W, Arts MP, Vleggeert-Lankamp CLA. Interspinous process device versus conventional decompression for lumbar spinal stenosis: 5-year results of a randomized controlled trial. J Neurosurg Spine. 2021 Dec 24:1-9. doi: 10.3171/2021.8.SPINE21419. Epub ahead of print. PMID: 34952518.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
