Marrow Cellutions and the Regen Med IQ Test
Copyright Disclaimer under section 107 of the Copyright Act 1976, allowance is made for “fair use” for purposes such as criticism, comment, news reporting, teaching, scholarship, education, and research.
Regenerative medicine and interventional orthobiologics are interesting because there is no standardized education for these topics in 99% of the residencies or fellowships. This creates an information vacuum that smart sales reps and device manufacturers can manipulate because they’re often where providers get their education. So today, I’ll challenge patients and providers with a Regen Med IQ (RM-IQ) test by reviewing the latest bit of misinformation on a device I have blogged on before, the Marrow Cellutions trocar. We’ll learn about low-quality, and high-quality bone marrow draws by reviewing the company’s latest white paper, and the sooner you spot the problem with that data, the higher your RM-IQ! Let’s dig in.
The New Marrow Cellutions White Paper
Marrow Cellutions is an expensive trocar made by Cervos Medical that costs about $1,200 compared to the average Jamshidi trocar, which costs about $35. A trocar is a specialized needle that a doctor uses to draw bone marrow aspirate (BMA). Why the high price? The company claims that this suped-up trocar can pull more mesenchymal stem cells out of the bone marrow due to its unique design with fenestrated sides and a closed tip. If that were true, the device could be worth more than a Jamshidi. Hence, the company has produced a series of white papers that have purported to back up that claim. More on those later as well as some of our collected data on this device.
The new white paper was posted all over social media this past week:
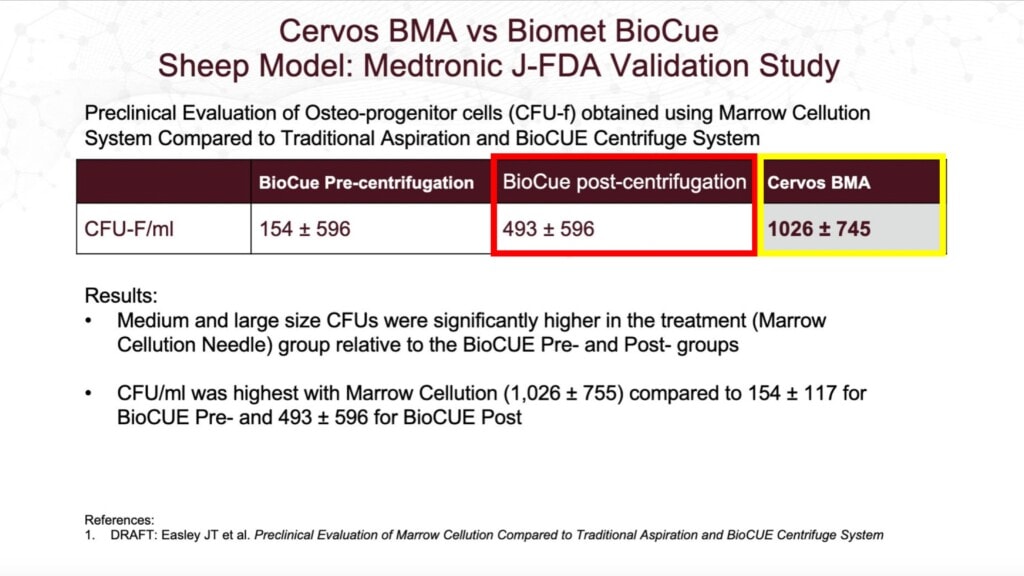
This white paper purports to show that the CFU-f was more than double for the Marrow Cellutions device (in yellow here and labeled “Cervos BMA”) when compared to the BioCue (red) after that system centrifuged marrow taken with a regular Jamshidi trocar and processed it using that kit. At first blush, this looks very impressive, given that the Marrow Cellutions didn’t need to centrifuge the BMA to get the higher CFU-f.
VERY HIGH RM-IQ–This is your first RM-IQ stopping point. Your score is much higher than average if you already know the potential problem with this study and if you already know what a CFU-f is and why it’s important.
What the Heck Is a CFU-f, and Why Is It Important?
A CFU-f is one way to measure the mesenchymal stem cell (MSC) content of the bone marrow concentrate (BMC) used in routine procedures. To get that number, a lab takes a small sample of the BMC and plates it in culture. Over 7-10 days, the MSCs adhere to the plastic flask, which are later stained, and the colonies counted. The number of colonies per unit volume of BMC is the CFU-f.
- A higher CFU-f means more MSCs in the BMC.
- You can NEVER compare CFU-f counts between labs. There are too many variables in plating, culture, and counting methods to make this a universal dose metric that goes beyond comparative research performed in one lab.
- CFU-f has been tied to clinical outcomes in papers published by the Regenexx research team and others (1-3).
The fact that the CFU-f count in the Marrow Cellutions device is higher than the other kit is good, right? Not so fast.
It’s All About the White Paper Methods
The first bit of data you should have asked for when seeing those methods is the technique used to draw both samples. Why is this important?
We have known for many years that how you draw bone marrow matters. For example, if you stick a trocar in the iliac crest and draw out one big pull of bone marrow aspirate (looks like thick blood), then you will artificially depress the number of MSCs in the sample (4-6). Why? Because the marrow space is jacked into the peripheral circulation. While bone marrow has lots of MSCs, the peripheral blood is very MSC-poor. So if you draw a large volume from one spot of the marrow, there will be lots of MSCs in the first few ml of BMA, less in the next few ml, and then much less from there on as that last part of the pull in mostly blood and not BMA. Hence, if you want to maximize MSCs in the marrow, you need to draw small amounts of marrow from many different spots. In fact, this is one way that most patients getting a BMC procedure get ripped off by their doctors, as the patient has no way of knowing if the doctor is leaving lots of cells in the marrow and drawing few because they want to get perform a quickie procedure.
The Marrow Cellutions device uses the small volume technique drawn from many spots. Hence, just using that technique, without any effect from a magic $1,200 device, will get you a better MSC-rich injectate. So the million-dollar question is whether the Marrow Cellutions and the regular Jamshidi that can be found in the BioCue kit, when used the same way, produce similar CFU-f values.
Finding the Data From the White Paper
The Marrow Cellutions advocate physician who posted the white paper on Linkedin failed to include any supporting data or methods. I then reached out to the veterinarian at CSU who performed the study for more info, but I never heard back. Why did a Vet perform this study? It was performed on sheep. Why test human 510k cleared devices on sheep? Great question, as we have performed many of these small studies in our consented patients, but most centers don’t do enough of these BMC procedures a month to make that practical.
I finally realized that this abstract/poster was presented at the Orthopedic Research Society Annual Meeting, so a search under the author’s name on that conference website yielded this complete abstract (click to read the PDF):
We see this in the methods:
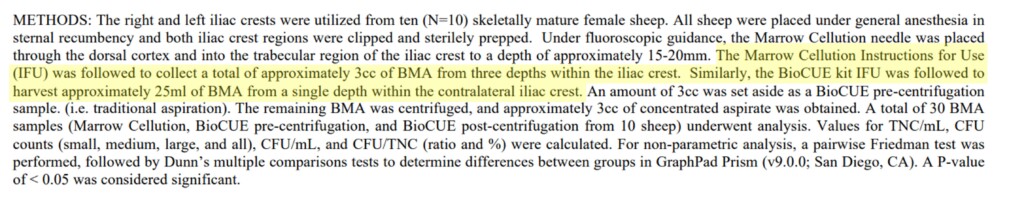
So only 3 ml (cc) of BMA was taken by the Marrow Cellutions system in 1 cc increments, and the Jamshidi used for the BioCue kit drew 25 ml all from one spot. Hence, this study presented at ORS isn’t worth the digital paper it’s printed on, as no matter what systems were used, the smaller 3 ml draw would always have many more MSCs per ml than the 25 ml draw. Hence, the study was designed to compare apples and oranges and then claim that apples were better than oranges.
The Prior Marrow Cellutions Research and Our Study
Many years ago, after seeing many physicians fall for the expensive Marrow Cellutions device without understanding the questions they should have asked, I did several blogs on the problems with the existing white papers (see blog 1 in 2017, blog 2 in 2018, and Blog 3 in 2019). Finally, we bought several devices ourselves because if this $1,200 doohickey could perform as described, it may have been worth purchasing them in bulk for our national network of physicians. However, we didn’t make the same mistakes as the previous white papers and the one I highlighted above. We used the cheap Jamshidi to draw small amounts of marrow from many different sites, which is our usual technique, and compared that to the Marrow Cellutions device. This is what the CFU-f data showed:
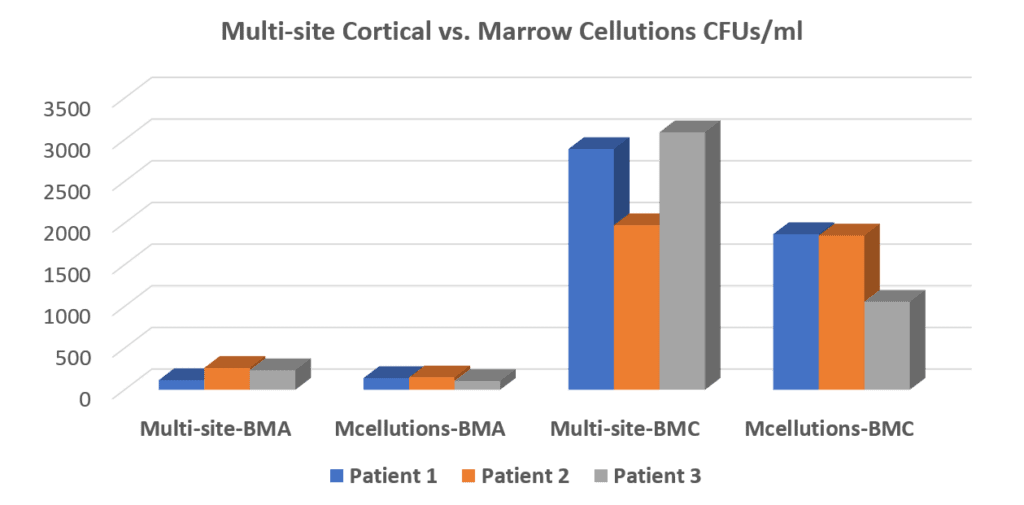
The chart above shows the CFU-fs produced by our usual multi-site BMA technique in the first column. The second column from the left shows that the Marrow Cellutions did worse than our usual draw, producing fewer CFU-fs. In column three, you see what we usually inject into patients after the BMA from our standard draw is centrifuged to get the stem cell fractions concentrated. Hence, the Marrow Cellutions vs. Regenexx comparison is the 2nd vs. 3rd column in that Marrow Cellutions is used without centrifugation, and our BMC is centrifuged using our proprietary technique. The comparison wasn’t even close, with our BMC having about 10X more CFU-fs than Marrow Cellutions. In the fourth column, we centrifuged the Marrow Cellutions BMA to see if that gave a different result. Ultimately, we concluded that these very expensive trocars were not worth the money as it was the technique that counted and not the expensive piece of metal and plastic.
Grading Your RM-IQ
Here’s how you should grade yourself:
Very High-You knew about CFU-fs and what could be wrong with the latest Marrow Cellutions white paper.
High-You only knew one of the two above.
Above Average-You didn’t know the first two, but you know that the research supports that using a low volume multi-site BMA technique produces better BMC. You also needed to know that clinical research ties a higher CFU-f to better outcomes in orthopedic treatments.
Average-You didn’t know the above.
Below Average-You had to look up the term “mesenchymal stem cell”
The upshot? At the end of the day, IMHO, this most recent white paper on Marrow Cellutions is just another silly attempt at trying to mislead physicians who don’t have a high RM-IQ. Regrettably, for patients, that’s most of the physicians out there claiming to be able to do this stuff. However, hopefully, this blog increased your RM-IQ!
______________________________________________________
References:
(1) Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015 Jan;33(1):146-56. doi: 10.1002/stem.1845. PMID: 25187512.
(2) Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002 Dec;(405):14-23. doi: 10.1097/00003086-200212000-00003. PMID: 12461352.
(3) Berger DR, Aune ET, Centeno CJ, Steinmetz NJ. Cryopreserved bone marrow aspirate concentrate as a cell source for the colony-forming unit fibroblast assay. Cytotherapy. 2020 Sep;22(9):486-493. doi: 10.1016/j.jcyt.2020.04.091. Epub 2020 Jun 19. PMID: 32565131.
(4) Batinić D, Marusić M, Pavletić Z, Bogdanić V, Uzarević B, Nemet D, Labar B. Relationship between differing volumes of bone marrow aspirates and their cellular composition. Bone Marrow Transplant. 1990 Aug;6(2):103-7. PMID: 2207448.
(5) Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997 Nov;79(11):1699-709. doi: 10.2106/00004623-199711000-00012. Erratum in: J Bone Joint Surg Am 1998 Feb;80(2):302. PMID: 9384430.
(6) Fennema EM, Renard AJ, Leusink A, van Blitterswijk CA, de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthop. 2009 Oct;80(5):618-21. doi: 10.3109/17453670903278241. PMID: 19916699; PMCID: PMC2823327.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.

