Busting the Mythbusters
I’ve previously blogged on the Marrow Cellutions device that purports to be so good at drawing stem cells right out of your bone marrow that the bone marrow aspirate doesn’t need to be centrifuged to concentrate these cells. We were intrigued a few years ago by the claim, so we paid for several of the devices and tested them. Regrettably, our tests couldn’t confirm that the device worked as advertised. Now the company has produced a Mythbusters series of videos that seem to be more myths themselves, so today we’ll bust the Mythbusters.
The MC Device
The Marrow Cellutions device (aka Ranfac or Maxx Regen device) is a trocar used to draw bone marrow aspirate (BMA). That’s the liquid portion of the bone marrow that contains stem cells. Typically, the doctor will remove this stuff that looks like blood (bone marrow aspirate, or BMA) and then centrifuge it to isolate the stem cell fraction. However, the makers of this device claim that you can concentrate the bone marrow just by drawing it with their device and get rid of the centrifuge. This advance would be revolutionary if it were true.
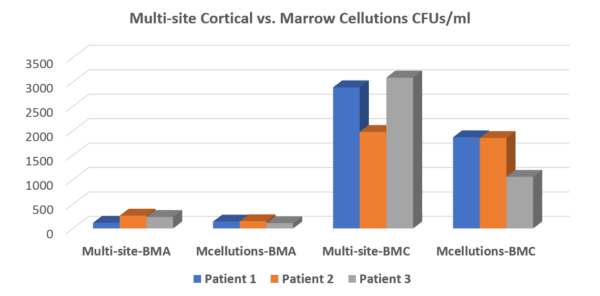
Based on this controversial claim, we tested the Marrow Cellutions device two years ago and found that it didn’t work as advertised. Below is the graph showing that the stem cells in our standard multi-site draw using a legacy device were more plentiful than in the Marrow Cellutions draw (first group of three compared to second with a higher bar representing more stem cells). Once we centrifuged the marrow using our protocol (the third group of three or “Multi-site BMC”), there was no comparison. For example, comparing that data below with the second group of three (which is what the Marrow Cellutions device produced) shows just how poorly the device performed.
At the same time, Dallas orthopedic surgeon Don Buford also tested the device and had similar results. Hence, we decided not to buy this device in bulk for our network providers to use. That, of course, hasn’t dissuaded the company’s army of sales reps from continuing to push doctors to use it. In fact, the most recent push has been a video series entitled “Myth Busters.”
The Orthobiologics Mythbusters Series
The company that makes this device has now created a new series of videos to educate providers. The vast majority of the speaking in the series is not accomplished by a physician or a PhD scientist, but by a business guy with a four-year degree in economics. That’s my first, “huh?” A few years back, I was on the conference committee for an orthobiologics conference, and we asked the company that makes the MC device if they could provide a medical doctor or PhD to speak on the pros of their approach to counter a speaker on the other side of the argument. They tried to offer this same speaker, Mr. McGillicuddy, the CEO of the company. I wouldn’t allow a guy with a four-year economics degree to get on the podium to speak about hardcore science at a medical conference. They also tried to offer Ms. Kashner, a physician assistant (PA), who also appears in the video. I also didn’t think that a PA was as an appropriate speaker for a medical conference focused on the science of orthopedic stem cell treatments. Hence, it’s unclear to me why medical doctors are being educated by a business guy and a PA.
Now that we know who’s presenting this data, let’s take the video series one video at a time. I’ll review two of the six today and hopefully be able to tackle the others later.
Myth #1—Red Blood Cells Are Negative to the Regenerative Response
In this video, Mr. McGillicuddy (Mr. M) discusses three cardiology papers that had different conclusions correlating the number of red cells in bone marrow concentrate and results. The issue he’s bringing up here is that some in the orthobiologics field think that “red PRP” (a concentrated platelet mixture with red and white blood cells) causes more of an inflammatory response and produces less robust outcomes than PRP that is amber in color and that has far fewer red and white blood cells. In discussing this, Mr. M uses three heart disease-treatment papers where they varied the processing of bone marrow (at one point he mistakenly says PRP). He concludes that red cells have no impact on the outcome and that the less we do to the biologic, the better.
So what does this have to do with using red cells in PRP or bone marrow in orthopedics? I have no idea. The only extensive data that’s available in that arena is from research on PRP. Here are a bunch of studies from the orthopedics literature that show that excluding the red and white blood cells worked better than keeping them:
-Adverse reactions in knee OA joint injections not directly related to WBC concentration, LP PRP outperforms LR PRP.—Am J Sports Med. 2016 Mar;44(3):792–800.
-LR PRP stimulates a pro-inflammatory environment in fibrin scaffolds that hurt fibroblast and osteoblast proliferation. PLoS One. 2015 Mar 30;10(3):e0121713.
-LR resulted in significant synovial cell death and pro-inflammatory mediators in-vitro, whereas LP did not. Am J Sports Med.2014 May;42(5):1204–10.
-LP caused better chondrocyte proliferation in-vitro. LP caused anabolic ECM production, LR caused catabolism—J Bone Joint Surg Am. 2014 Mar 5;96(5):423–9.
-Knee OA-LP outperformed LR on functional scores—Am J Sports Med. 2016 Mar;44(3):792–800.
-LP caused less tendon inflammation, but similar cellularity-animal model—Am J Sports Med. 2012 Jun;40(6):1274–81.
On Mr. M’s comments that older patients likely have more significant flare-ups (inflammaging) when red and white blood cells are present, I would agree. However, I have to ask what a guy with an economics degree knows about the clinical use of different types of PRP.
Myth #2: How and Where You Draw from Has No Impact on Quality of Marrow
Mr. M starts out discussing how stem cells in bone marrow may be better if they are internal to the bone rather than on the periphery. I read the reference he cited to support that idea and couldn’t find that discussed; maybe you can locate it? He then goes into the idea that small draws across a large geography (taking less volume from more spots) gives you more stem cells. This bone marrow-draw technique is correct, as I have discussed numerous times in this blog and my published book chapters. Keep that fact in the back of your mind as it will become important later.
Mr. M gets into the idea that having a side port on the trocar you use for a BMA helps bone marrow yield. He gives no reference, but here is a study that showed that side ports (fenestrations) on the trocar decreased the number of stem cells.
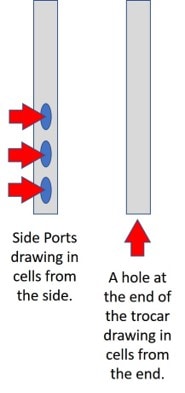
Much of Mr. M’s discussion relies on the idea that the Marrow Cellutions trocar he sells has side ports and a closed tip, which is unlike a standard Jamshidi trocar with an open tip with or without side ports (see left image). So, does a closed tip at the end of a trocar justify the 20X pricing on his doohickey? You read that right: the Marrow Cellutions trocar is about 20 or more times the cost of the standard-of-care device. Let’s dig deeper.
As you can see to the left, he believes these side ports pull in more of the type of cells you want. He then goes on to discuss why research shows that this is true, except the research he shows proves nothing of the sort. For example, in the video, he purports to show that more stem cells were obtained with his device, but he violates the rule that he told us about in the first part of the lecture. Remember, drawing lower volumes gets you more stem cells, and higher volumes net fewer stem cells. In the video, he shows multiple charts and graphs where he compares a low-volume draw with his device to a higher-volume draw with another device. Hence, we would expect the higher-volume draw to have fewer stem cells per unit volume, which is what we find in the table and flow cytometry graphs he presents. However, Mr. M believes that this comparison proves his device works. Nope, this only proves that drawing lower volumes is a better idea, whether this is with this expensive device or with a standard device.
Mr. M then gets into a slide that shows the results of an amended white paper published by Harrell. I critiqued this advertising study in 2016. Here Mr. M seems to be trying to fix the volume problem stated above. While the original Harrell paper made the same volume error, this appears to have been amended since my blog called Harrell out for comparing the results of a bad, high-volume draw to a good low-volume draw with the Marrow Cellutions (MC) device and then claiming it was the device that made the difference. The new amended study seems to have added a single side-to-side comparison where a 5 ml draw on one side with a “legacy” device was compared to an 8 ml MC draw on the other. Mr. M suggests that since Dr. Harrell’s white paper showed a higher number of stem cells with the MC device here in this single patient, this is proof that MC works. The problem was that we couldn’t independently prove that in three patients, and Don Buford (a Dallas orthopedic surgeon) couldn’t prove it either after testing several additional patients. In fact, in all of the published white papers produced by this company with data from tens of patients, this is the only patient where I have seen a side-by-side comparison performed at a similar volume in the same patient.
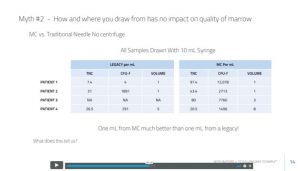
Maybe this is answered on the next slide? We now see a table (above) with four patients with low-volume draws. We only know that one of these was compared side to side, which is the patient discussed above (that’s patient four here). The number of stem cells in the table is in the CFU-f column. So are patients 1–3 the same patient, and is this data from the bone marrow that was harvested from side to side (MC device on one side and a standard Jamshidi on the other—here called “legacy”). That’s where things get a bit murky. I tried to ask Mr. M directly, but he refused to answer. Here was his response:
“Let me work on the following: A) Getting a more robust set of data published. Prelim feedback from editor of draftis encouraging so hopeful next six months. B) organize an open forum discussion after publication.”
Huh? He seems to be admitting that sufficient direct comparison data doesn’t exist yet. So let me get this straight. You have sold a device for years that costs 20–30X more than the legacy device used for decades on the main premise that it can get more stem cells. However, the only experimental design that would prove that point is a direct side-to-side comparison in the same patient where the same low-volume draw method is used. However, you have little such data, so you hope to collect some to support that it works? Shouldn’t we find out it works before we make and sell the device?
To see how deep this company has dug this hole, let’s jump into the data from the table above. What are the issues? First, TNC means total nucleated cells. That’s the total number of cells in the sample. Next, notice that patient 1 has a TNC of only 7.4 (million) per ml with the legacy draw and a TNC of 97.4 with the MC device. This TNC number makes no sense on the legacy side of patient 1, as it is very different than the other draws in this table. It also doesn’t benchmark well against any of the other data that Mr. M has presented in the whole video. In fact, no other patient described in this entire presentation has a TNC under 15 million TNC/ml in the legacy draw. Hence, this is likely either a draw-technique error or a lab sampling error.
Throwing out patient 1 of this table leaves us a grand total of two patients where the MC device has compared itself to a legacy device where a similar or the same low-volume-draw method was used. We only know that this MAY have been done in these two patients, but we can only confirm that this was done this way for sure in one patient. Even comparing these two patients causes problems.
Remember where Mr. M said that the number of CFUs (stem cells) as compared to the TNC (total nucleated cells) was important. If the MC device is reliable and reproducible, it should be able to pull a consistent number of stem cells versus nucleated cells. That ratio should stay the same or approximately the same or at least be in a somewhat narrow range. In fact, Mr. M made a huge deal of this ratio concept in his video. That’s not what we see when we compare patients 2 and 4. For example, in patient 2, the device is pulling out about the same number of stem cells versus nucleated cells. In the case of patient four, however, it’s pulling 5X as much? Those disparate data points don’t fit with the idea that the device can produce reliable results in many patients. Again, we have data that isn’t internally consistent and readily explainable.
So is this the range we can expect, from zero advantage to 5X? With only two patients that maybe, kind of used the same draw method, your guess is as good as mine. Meaning, for us to have any idea of whether what we’re seeing is random chance or an actual effect, with this kind of huge range, we would need to study a large number of patients to obtain statistical significance.
The Tail Often Wags the Dog in Medicine
One of the problems with medical devices that go through the 510K FDA pathway is that few, if any, start with any clinical data. Meaning, for the MC device, all Mr. M’s company needed to show the FDA to get to market was that it was “substantially equivalent” to an existing device, which in this case is the Jamshidi trocar. In particular, no clinical or lab data was required. That’s why it’s called “FDA cleared” and NOT FDA Approved. In this case, as you can see, while many white papers have been published to try to back into the idea that the device obtains more stem cells, no convincing data exists to prove that point. However, now that there is a small army of sales reps selling and doctors invested in using the device, there is enormous pressure to prove it works. Again, the tail wags the dog.
The upshot? Today I’ve busted the first two Mythbuster series videos (#1 and #2). What’s fascinating here is that instead of listening to a PhD or physician lecture us on orthobiologics principles, we’re listening to a salesperson who has a four-year degree in economics. If you’re a physician, this should instantly get your radar up about carefully reviewing what’s being said. What I’ve shown here today is that while all of this looks highly technical and credible at first blush, a more in-depth analysis doesn’t support that the Marrow Cellution device is capable of getting more stem cells than just a proper draw performed by an experienced physician using a “legacy” (standard of care) device. At the end of the day, that’s what the two independent tests of this device have shown. More disturbing is that Mr. M has refused to answer the direct questions above that I have put to him about the head-to-head comparison data. Maybe I’ll get the answers at some point? In the meantime, I hope to have time to go through the rest of this series in future blog posts.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.