Riverside Biologics Health Review: At Home “Stem Cells”?

I get sent stuff by patients all the time. This week a patient reached out about Riverside Biologic Health’s IV and injection “at home” umbilical cord “stem cell” service. Hence, let’s dig in.
The Emails
I received two unsolicited emails from a patient describing himself as an advocate for Regenexx:
“High Tina, please pass on my request to dr. Centeno about the back ground of Riverside biologics, and also the director Dr. Neil Riodan. I attended one of their sales meetings and listened to some fantastic claims of which most were lies. One item of interest I did not understand was that the DNA becomes inactive when the cells are extracted from the fetus because of the HLA protein so there is no possibility of rejection. I tried to research this but did not get a clear understanding. All the other claims were of course the Wild West. The marketing was interesting in that in my state on rep would send post cards in each city or town inviting people to come to the meetings for healing.”
“Good morning doc, I remember you mentioning this outfit in one of your blogs but I would like to know more about dr. Neil Riodan and the macenna institute. I attended one of their marketing meetings in ohio and it was the wild west of stem cells. The speaker claimed that riodan had 40 patents and 70 publishings to back up all of his claims. I wont bore you with all the claims, you have heard them before, but he made one remark about no possibility of rejection using foreign live cells because of the HLA protein. I did not know what he was talking about and tried to research it and did not find anything to explain it. I will add that the procedures range from $4000 to $14000 administered in your home by a PA. At the end of the meeting I confronted the speaker about all the stuff I learned from you and he said Regenexx is a bully. Is there any truth about the HLA protein?”
What Is Riverside Biologic Health?
That’s a good question. On the website, they claim to offer both “Regenerative Cell Therapy” and be a “Leader in Joint Pain Therapy”. Their website is pretty nebulous, but let’s start with this “coverage” map:
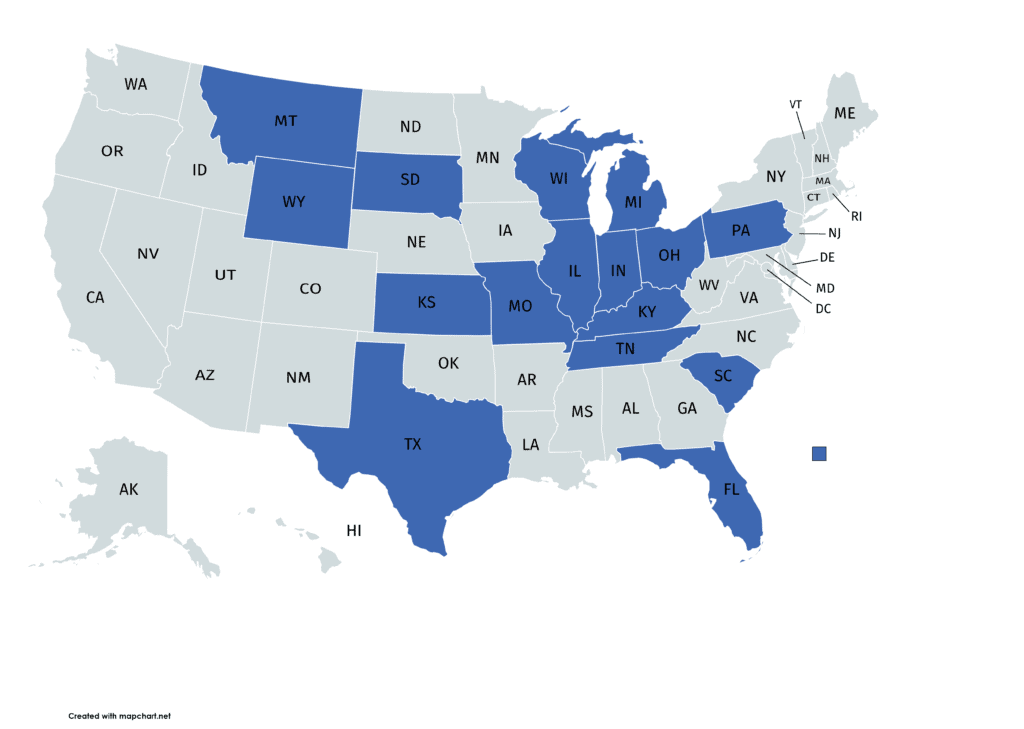
That word choice is interesting. Notice how instead of saying that the company has affiliates, offices, or clinics in these states, it uses the term “coverage”. In fact, you can’t find out anything about what this coverage entails. However, a familiar name does pop up here on their website: Scott Gray, DC.
I’ve blogged on Scott Gray, DC before. He is/was the CEO of Stem Cell One and Gray Marketing Enterprises. What is “Stem Cell One”? Another chiropractic clinic that has billed itself as “Leading Umbilical Stem Cell Therapy in Marion, Ohio”! Scott also owns a company called “Gray Marketing Enterprises,” and he used to state on his LinkedIn page, “Dr Gray also published a book helping chiropractors help more patients; The Ultimate New Patient Machine.” That profile, after being discussed in a prior blog has now been deleted.
I’ve also blogged before on Biologics Health, a company associated with Scott Gray. When you Google Biologics Health now and Scott’s name, Riverside Biologics Health comes up, so I have to assume that Scott added the word “Riverside” to this company.
A Home “Stem Cell” Service?
Last year when I reviewed Scott’s at-home umbilical cord “stem cell” service called “Biologics Health”, it was pretty much the same business now being described by this patient. A nurse would come to your home to give you an IV of umbilical cord “stem cells”. It also looks like that same nurse will perform a joint injection, but perhaps that’s performed in a clinic? This was all very concerning at that time, as any such procedure should be performed in a room that looks like this:
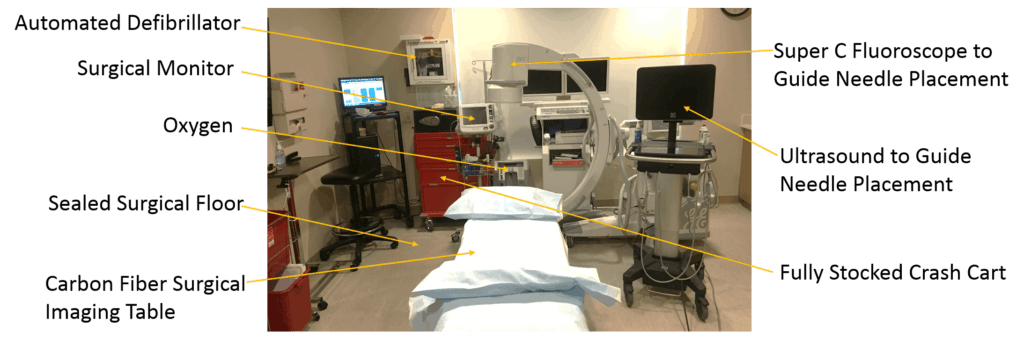
And not like this:

Another problem was that the research back then and since, given our new paper published in the American Journal of Sports Medicine and those papers published by others, showed that commercially available umbilical cord preparations had no living stem cells (1-4). It looks like since the FDA crackdown Scott has scrubbed this new website of the phrase “stem cells”, but based on the patient’s communication above, that’s still central terminology used in their seminars. Frankly, it’s amazing that the FDA has yet to stop this one, as IMHO I can think of few things that sound more innocuous, but in my opinion, are more dangerous than an IV umbilical cord infusion. Let’s dig into that topic.
Why IV Umbilical Cord Cell Treatments Are Risky
First, as discussed above, none of these umbilical cord products sold in the US have any live and functional mesenchymal stem cells. The research in this area has made that clear time and time again. However, a few of these products do have live and dying cells which are foreign tissue. When umbilical cord blood treatments are used in pediatric cancer cases, these are HLA matched to the patient (5-7). Why? The patient’s immune system will reject the cells, however, there will be less rejection if the cell surface markers on the umbilical cord cells are a close match to the patient’s own markers. However, I have yet to see a company using this stuff that matches the patient to the product.
What can happen when mismatched umbilical cord cells are injected into a body? A severe rejection reaction known as “Graft vs. Host Disease” (GVHD) (7). GVHD can be mild with rashes and feeling sick or it can be severe with organ failure. I know this one personally, as I’m the medical expert on several legal cases where umbilical cord tissue injections were given in chiropractic and medical offices, and in my medical opinion, caused GVHD. In one case, a poor elderly man went into renal failure.
How about the salesperson’s statement above? This what the patient relayed was said:
“One item of interest I did not understand was that the DNA becomes inactive when the cells are extracted from the fetus because of the HLA protein so there is no possibility of rejection.”
Is this scientifically accurate? Nope. If you have live cells that are dying, the tissue will be read as a foreign invader by the patient and an immune response will be mounted. Now if the manufacturer made a conscious effort to kill all of the cells, that could help. However, the handout given out at the seminar by Riverside Biologics Health website says:

That certainly sounds like Riverside is claiming live cells. The rest of the brochure talks about how stem cell numbers decline with age (not actually accurate in the way portrayed, see this blog for more information).
Who gave this medical advice? Scott Boyer, a guy with a 4-year communications degree and Dale Carnegie sales training. Based on his Linkedin profile, Scott is a professional salesperson experienced in giving sales presentations and NOT a medical or scientific expert.
My Published Research Paper Is Used?
One of the bait and switch tactics used by these “stem cell” outfits is that they post research that has nothing to do with the product they’re offering to lend a veneer of credibility to their sales pitch. Riverside Biologics Health does that as well. Not a single paper listed has anything to do with the off-the-shelf umbilical cord tissue product they’re using. Even nuttier, they have listed one of my earliest research papers on using culture-expanded bone marrow stem cells in a knee, which again has nothing to do with dying umbilical cord tissue in a bottle.
Neil Riordan
It’s clear from the patient that Neil Riordan’s product is being used in this venture. This is a product that I have blogged on called Signature Cord where the manufacture has claimed that it contains live and functional mesenchymal stem cells. Again, despite this past claim, there is no credible scientific data that this umbilical cord product contains any live and functional MSCs.
I know Neil. He has a Ph.D. and is a Physician’s Assistant. He has published a slew of things about his live stem cell product being used at his clinic in Panama but has yet to publish any randomized controlled trial on those treatments. In addition, I know of no studies at all on the US product called Signature Cord, which is what’s being used here. Hence, in my opinion, this is more bait and switch on the part of Riverside.
Where Is Riverside Biologics Health?
Given that Scott’s Linkedin profile was deleted, I decided to see if I could find a corporate HQ for either Biologics Health or Riverside Biologics Health. That led me to a corporate registration agent in St. Pete, Florida, but no offices could be found. IMHO, that’s par for the course here, as Riverside is a sales company run by a chiropractor whose career has focused on increasing the revenue of chiro practices. Meaning that Riverside is not a biotechnology venture nor is it a chain of clinics staffed by physician specialists.
An FDA Certified Lab?
The website states, “the products of conception (amniotic fluid, umbilical cord, placenta, and accompanying materials) are discarded. In this case, it’s donated by the mother, with no harm to the baby. The materials are transferred immediately via a sterile container to a nearby FDA certified lab.” Is there such a thing? Nope. The FDA doesn’t certify labs that process birth tissues. You can register your lab via a free registration system (Tissue Establishment Registration), but that only tells them where to go if they want to inspect you. That doesn’t mean they have certified or approved your lab.
The FDA Action
One of the reasons that there has been a name change, adding the word “Riverside” to “Biologics Health” may be that the company received a letter from the FDA. This letter states exactly what I have reviewed, that Riverside Biologics Health is violating federal law by delivering umbilical cord IV treatments to patients. Has that stopped the company? Apparently not as they have given seminars after that date.
The upshot? At the end of the day, IMHO, sending nurses to homes to inject umbilical cord tissue is not Kosher. I hope the FDA continues to act on this one.
______________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.
(5) Eapen, Mary et al. Mismatched Related and Unrelated Donors for Allogeneic Hematopoietic Cell Transplantation for Adults with Hematologic Malignancies. Biology of Blood and Marrow Transplantation, Volume 20, Issue 10, 1485 – 1492. https://www.ncbi.nlm.nih.gov/pubmed/24862638
(6) Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923–1930. https://www.ncbi.nlm.nih.gov/pubmed/15191952
(7) Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood Jul 2014, 124 (3) 363-373; DOI: 10.1182/blood-2014-01-514786

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
