Seeing Patients in Our Groundbreaking Shoulder Rotator Cuff Stem Cell Trial
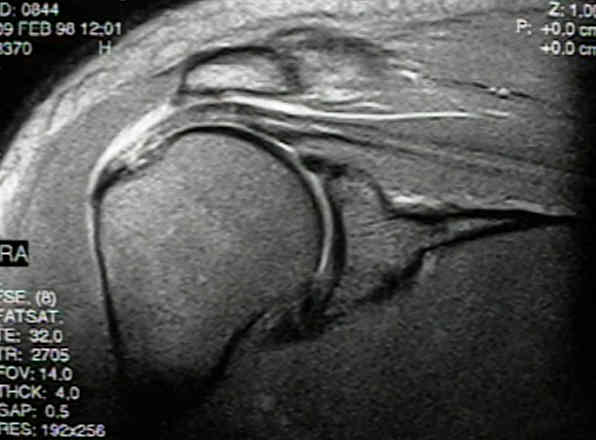
I examined a patient yesterday in clinic for our shoulder rotator cuff stem cell trial. She was randomized to wait three months and has been engaged in home exercises for her full thickness rotator cuff tear. Since she still has issues with returning to full activity, she’s been cleared to get the actual stem cell treatment. This brings up an important point, this randomized controlled trial is a cross over design, meaning that if you get chosen to be in the control group like this patient, you only need to wait a few months before getting the actual treatment. This is far more patient friendly than a placebo study, where you won’t know if you will get the real treatment or a fake placebo therapy. Sometime soon we will map her shoulder rotator cuff using 3D ultrasound volume acquisition (an MRI like technology) and meticulously place stem cells into her rotator cuff tear using real time ultrasound guidance. Because her tear is such that her rotator cuff is still largely intact, she doesn’t need the same type of immobilization that a surgical patient will need.
The upshot? If you know somebody who has a shoulder rotator cuff tear who is interested in helping research or otherwise can’t afford a Regenexx procedure, have them contact our study coordinator at studycandidate@regenexx.com. The procedure is provided at no cost.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
