Should You Get an SI Fusion?
SI joint fusion is all the rage right now. My last in-depth update on this topic was in 2019 and 2020. My conclusion back then was to ask why we were fusing the SI joint when we can easily treat lax ligaments through injection with far fewer complications. However, since then SI joint fusion has continued to explode, driven by better insurance coverage. Hence, it was time for another deep dive into this topic.
What is SI Joint Fusion?
Fusion just means that a surgeon installs a screw, rod, plate, cage, or bone to make a part of the spine that was built to move “fuse” and not move. The SI joint has been fused on and off since the beginning of my career in the early 90s. Back then, it was eventually determined that the procedure was not very effective and caused more problems than it was worth. However, beginning around 2011 SI fusion made a distinct comeback. It’s that encore performance that is the topic of today’s blog.
In SI fusion 2.0, the procedure was made “minimally invasive”. That meant that it was reswizzled such that a non spine surgeon could use a device to install a screw or another device through the joint. That meant a MUCH bigger physician user base in that now interventional pain physicians could perform the procedure. That decision is responsible for the explosion in the use of this procedure.
My Infographic
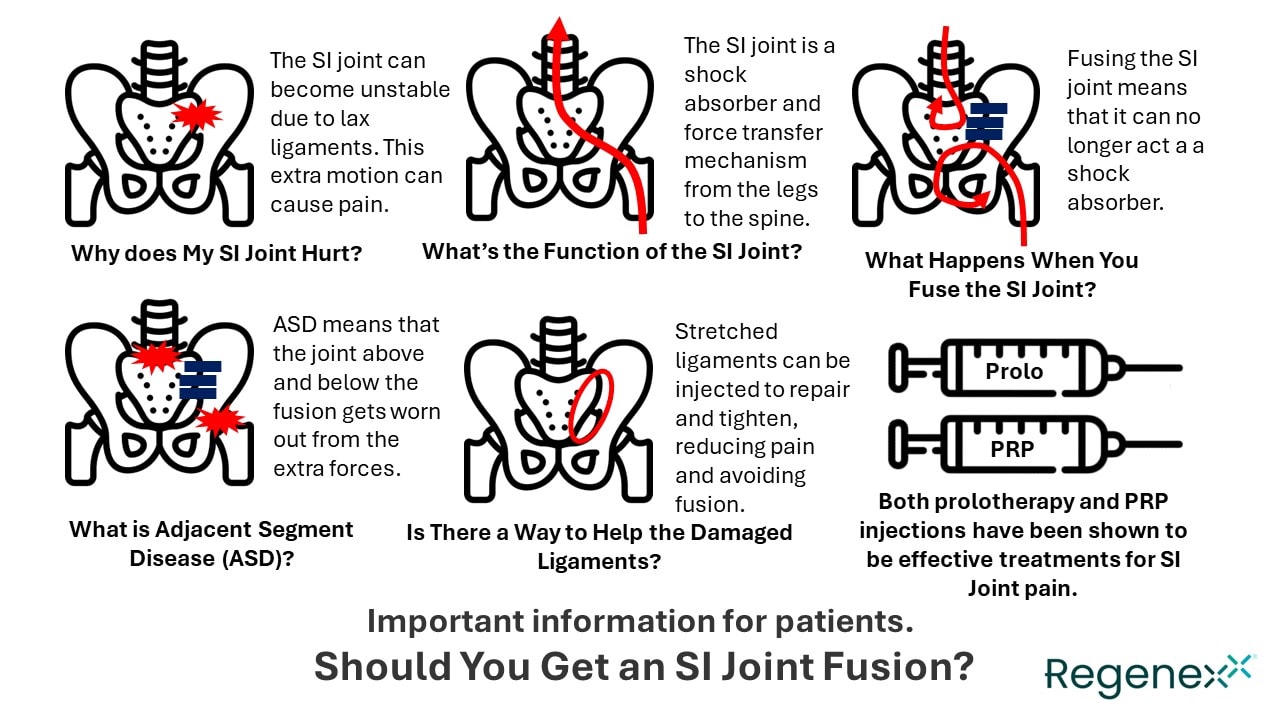
For this one, I’ll start with the simple stuff. I was on a roll creating many infographics about orthobiologics when I got the inspiration for the one shown above. That inspiration came from the large number of my colleagues who are now performing this procedure and the need to educate patients simply.
First, the SI joint lives between the ilium (pelvis) and the scarum (tailbone). When it hurts, the most common cause is instability. This means that the ligaments that are supposed to hold the joint together get stretched or damaged. This leads to instability or excessive motion which leads to wear and tear and chronic inflammation and pain.
The SI joint is viscoelastic. That means that it acts as a force transfer mechanism between your legs and spine and doesn’t move like a hinge joint such as your knee.
What happens when you fuse the joint? That viscoelastic force transfer mechanism is disrupted. Now the forces that come up from the leg concentrate at the hip. The forces that come down from the bending of the spine when you walk are not transferred in the same way to the leg. Instead, they concentrate on the lower lumbar area. What happens over time when we create this type of adjacent segment disease (ASD)? The hip and lower back are more likely to wear out in most patients.
Despite ASD being a well-known phenomenon in all types of spinal fusion, the SI joint fusion community has never studied this issue in living patients who have received these implants. However, based on other fusion devices, you can lay a pretty big bet that at some point the clinical data will show ASD effects in the hip and lower back due to these devices.
Finally, we already have high-level randomized controlled trials that show that injections like prolotherapy and PRP help chronic SI joint pain (7,8). These are injections into the ligaments to strengthen and heal those structures.
Types of SI Joint Screws
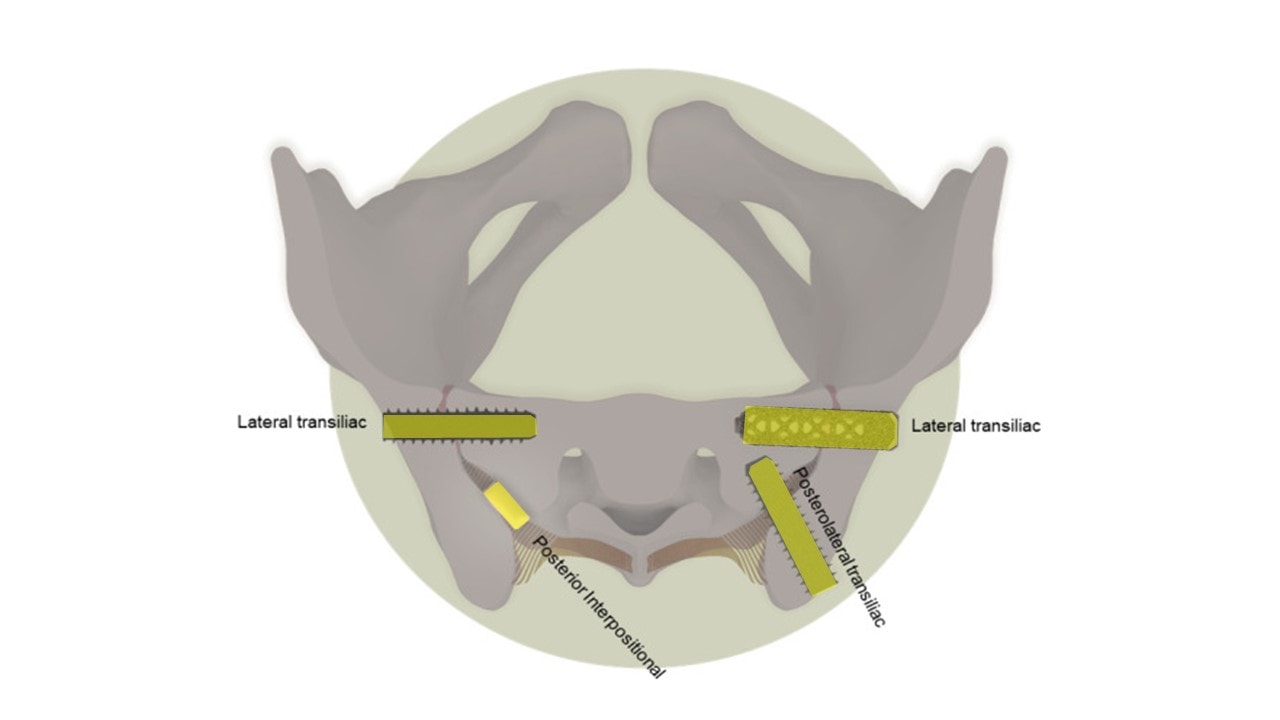
Adapted from Whang PG, Patel V, Duhon B, Sturesson B, Cher D, Carlton Reckling W, Capobianco R, Polly D. Minimally Invasive SI Joint Fusion Procedures for Chronic SI Joint Pain: Systematic Review and Meta-Analysis of Safety and Efficacy. Int J Spine Surg. 2023 Dec 26;17(6):794-808. doi: 10.14444/8543. PMID: 37798076; PMCID: PMC10753354.
I added the highlighting above from a review publication on minimally invasive SI joint fusion. There are four different types:
- A lateral transiliac screw that laterally traverses to iliac wing and sacrum
- A lateral transiliac cage that laterally traverses the iliac wing and sacrum
- A posterior interpositional dowel that fills up the posterior part of the joint space
- A posterolateral transiliac screw that traverses from the PSIS to the sacrum
The largest amount of outcome data is published on the lateral transiliac (LTI or iFuse device).
Literature Review
Here my focus is on randomized controlled trials with at least 1-2 years of follow-up, preferably 2 years.
There is an industry-sponsored randomized study published in 2017 with a 1-year follow-up comparing SI fusion to physical therapy (iFuse) (4). After a year, the difference between the two groups was a drop in pain of 2.8 VAS points (0-10 scale) compared to physical therapy. There were also more functional improvements in the SI fusion group.
There is a 2016 industry-sponsored randomized study with a 2-year follow-up that I have previously blogged on (SI-Bone) (6). While the SI fusion did reduce pain more than physical therapy or steroid injections, the reduction in opioid use for the roughly two-thirds of patients who took these medications was unimpressive. For example, at 24 months, 70% of the patients who were taking opioids and had an SI fusion continued to take opioids.
The problem with this data is obvious. Both of these are industry-funded studies. This issue was brought up by one of the review papers out there:
“Although outcomes have been widely studied, more studies, especially prospectively designed and those without industry influence be performed to elucidate the optimal management of patients with intractable SIJ pain.”
Big Procedure Means Big Sham Effects
The biggest problem with the efficacy data on SI fusion devices to date is that any surgical procedure is likely to have a massive sham effect. No study to date attempted to adjust for this by actually including a sham surgery group. On a positive note, there is a small 60-patient sham trial underway that’s underway in Oslo, Norway (3).
Complications
A 2022 study showed 1,115 reports of adverse events listed in the FDA MAUDE system for device complication tracking (2). 98% of these resulted in patient injury with malposition of the screws being the most common issue at 49.5%. Physician error represented 58% of these cases with revision surgery needed in 93%. The authors recognized that this likely represents the under-reporting of the total complications out there due to these devices.
Here’s a word cloud of those complications that I have previously created:

How under-reported are SI joint fusion complications? The studies paid for by the device companies generally state that only about 5% or fewer of the patients had significant complications. However, a non-device-sponsored study tells a different story (10). This data comes from insurance claims on complications from >400 SI joint fusion procedures. This complication rate was >3X the data reported in the company-sponsored study (4.7% vs. 16.4%)!
A common adverse event noted in this insurance claims research was “new spinal problems”. Let’s dive into that topic.
A device company-sponsored research study with a four-year follow-up is illustrative (9). When reading the fine print, I noticed this statement:
“In total, 114 adverse events were reported between years 3 and 4; however, none were rated as probably or definitely related to the study devices or index surgical procedure.”
Why did this statement stick out? Because this next sentence worries me:
“Many events indicated underlying degenerative disease associated with age and osteoarthritis (eg, hip, knee, shoulder, neck, and lumbar spine osteoarthritic degeneration).”
IMHO, this is an indicator that the ASD that I discussed above is indeed occurring in these fused patients and is being written off by the study investigators as unrelated to the SI fusion.
A Reasonable Decision Matrix Based on the Literature and Common Sense
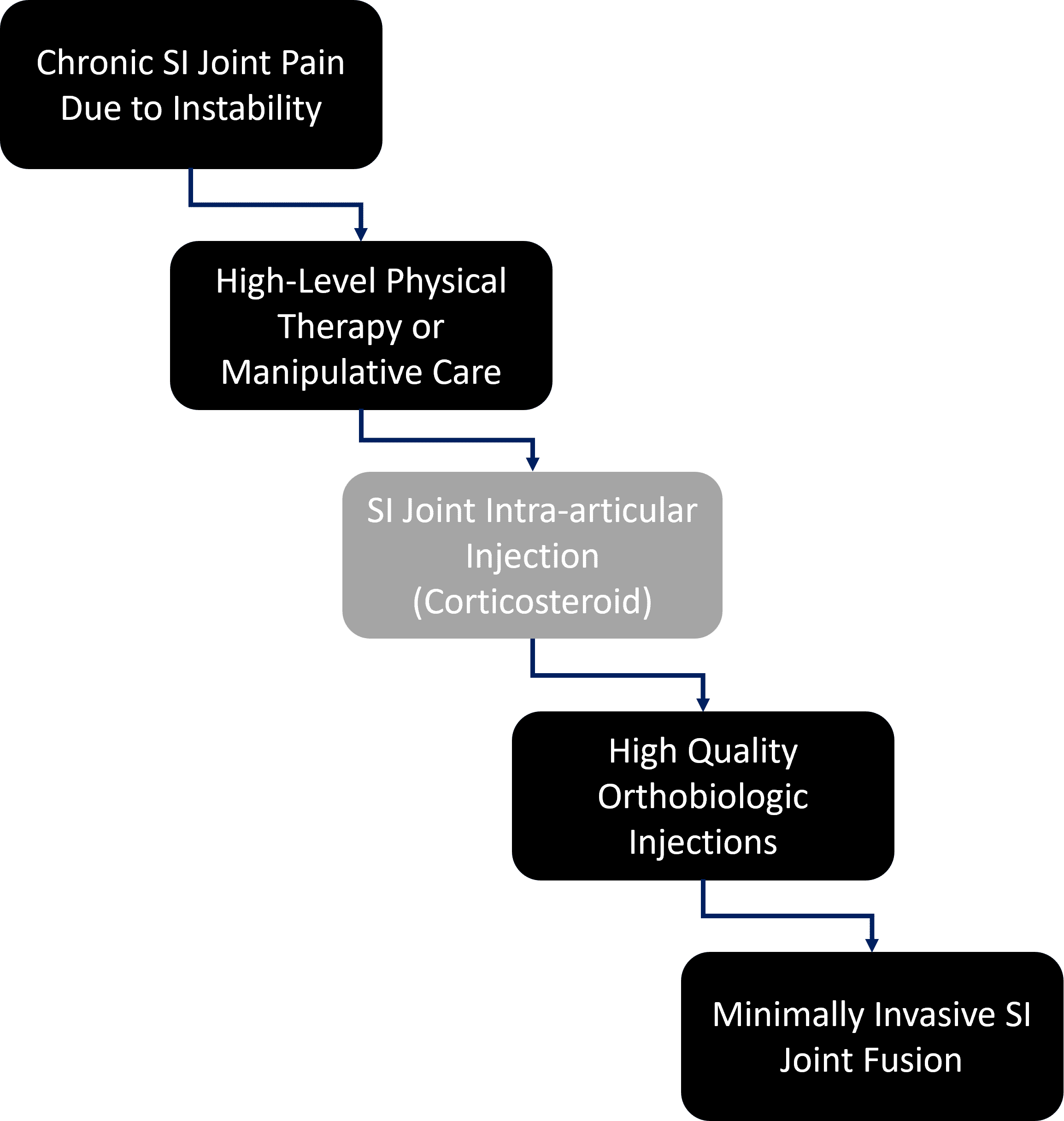
How do we protect patients? By exposing them to the least risk possible at each step in the clinical decision tree. Hence, in the above decision matrix, many patients will do fine with just physical therapy and never need any more care. While I’m no fan of high-dose steroid injections into joints due to the issue of known cartilage toxicity (which is why this step is greyed out), some patients will likely fall out at this step and recover. If that fails (or this step can be skipped in favor of orthobiologics), then in my clinical experience the vast majority of the patients remaining will be responsive to prolotherapy or PRP injections into the joint and ligaments. The very few patients with SI pain remaining after all of these steps will then proceed to a much more invasive SI joint fusion.
Is this what happens today? Nope, the orthobiologics step is almost always skipped over in favor of SI fusion. The physicians I talk to complain that since there is no insurance coverage for orthobiologics, it’s easier to just go to the fusion. This is despite exposing the patient to much more unnecessary risk. For the Regenexx network, this is less of a problem since we have about 2,000 private companies that will pay for orthobiologics.
The upshot? SI joint fusion is an option for a handful of patients who fail all other less invasive attempts to solve their SI joint instability. Because of complications, it is not a first-line option for patients failing PT and a steroid injection. However, like many things in medicine, insurance coverage drives clinical decision making, even when that means exposing the patient to additional risk.
__________________________________________________
References:
(1) Mehkri Y, Tishad A, Nichols S, Scott KW, Arias J, Lucke-Wold B, Rahmathulla G. Outcomes After Minimally Invasive Sacroiliac Joint Fusion: A Scoping Review. World Neurosurg. 2022 Dec;168:120-132. doi: 10.1016/j.wneu.2022.09.094. Epub 2022 Sep 26. PMID: 36174944.
(2) Rahl MD, Weistroffer J, Dall BE. Analysis of Complications in Sacroiliac Joint Fusions Using FDA 510(k) Cleared Devices. Clin Spine Surg. 2022 Apr 1;35(3):E363-E367. doi: 10.1097/BSD.0000000000001264. PMID: 35239289.
(3) Randers EM, Gerdhem P, Dahl J, Stuge B, Kibsgård TJ. The effect of minimally invasive sacroiliac joint fusion compared with sham operation: study protocol of a prospective double-blinded multicenter randomized controlled trial. Acta Orthop. 2022 Jan 3;93:75-81. doi: 10.1080/17453674.2021.1994185. PMID: 34694204; PMCID: PMC8815456.
(4) Dengler JD, Kools D, Pflugmacher R, Gasbarrini A, Prestamburgo D, Gaetani P, van Eeckhoven E, Cher D, Sturesson B. 1-Year Results of a Randomized Controlled Trial of Conservative Management vs. Minimally Invasive Surgical Treatment for Sacroiliac Joint Pain. Pain Physician. 2017 Sep;20(6):537-550. PMID: 28934785.
(5) Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, Cher DJ, Wine KD, Sembrano JN; INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016 Aug 23;10:28. doi: 10.14444/3028. PMID: 27652199; PMCID: PMC5027818.
(6) Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, Cher DJ, Wine KD, Sembrano JN; INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016 Aug 23;10:28. doi: 10.14444/3028. PMID: 27652199; PMCID: PMC5027818.
(7) Singla V, Batra YK, Bharti N, Goni VG, Marwaha N. Steroid vs. Platelet-Rich Plasma in Ultrasound-Guided Sacroiliac Joint Injection for Chronic Low Back Pain. Pain Pract. 2017 Jul;17(6):782-791. doi: 10.1111/papr.12526.
(8) Kim WM, Lee HG, Jeong CW, Kim CM, Yoon MH. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010 Dec;16(12):1285-90. doi: 10.1089/acm.2010.0031.
(9) Darr E, Cher D. Four-year outcomes after minimally invasive transiliac sacroiliac joint fusion with triangular titanium implants. Med Devices (Auckl). 2018;11:287–289. Published 2018 Aug 29. doi:10.2147/MDER.S179003
(10) Schoell, K., et al., Postoperative complications in patients undergoing minimally invasive sacroiliac fusion. Spine J. 2016 Nov;16(11):1324-1332. doi: 10.1016/j.spinee.2016.06.016.
Originally published on

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
