Stem Cell Institute of America Brochure Review
It’s been a while since I have blogged on the Stem Cell Institute of America. Mostly because I haven’t been seeing as much, but I get sent stuff all the time and this past week I was sent one of their brochures. It was so inaccurate that I thought it was worth a blog all by itself.
What is the Stem Cell Institute of America?
This chiropractic focused marketing group isn’t really an institute, but a sales operation for products and patient acquisition tools. They first came to my attention when local chiropractors who had zero business offering any injection-based therapy let alone investigational stem cells, started carpet bombing our local paper with ads for seminars. Soon I heard other doctors around the country tell me that the same ads were appearing in their papers. We investigated the group several years ago and found out all sorts of crazy stuff, check out that video below:
The group also uses the website www.americastem.com.
The biggest issues I have had with the group are:
- There is no place for mid-levels like nurse practitioners and physician’s assistants to be involved in delivering investigational orthobiologics care, especially in chiropractic offices.
- They use birth tissue products which are dead but claim that these are live and functional “stem cell” products.
- They often deliver simple injections that are often charged at some of the highest “all the traffic will bear” prices.
- They use aggressive seminars often focused on the elderly which use high-pressure sales tactics
- They use faked before and after x-rays to sell procedures
Is SCIA Still Active?
I can no longer find an active website for this group as of this morning as the one above says “Coming Soon”. This is despite this brochure still being in use. However, this brochure contains so many errors that are common in the space of chiropractors offering dead stem cells, showing it’s inaccuracies will help patients cut through the noise at not only SCIA clinics, but at many clinics.
The SCIA Brochure
I write almost every day, so I’ve had my share of misspellings or incorrect syntax go out there, as I often push the “Publish” button at 6:30-7:00 am after starting at 5-5:30 a.m. However, this SCIA brochure is a mess of misspelling and awful syntax. It’s hard to believe that someone allowed this thing to be sent to a printer.
However, let’s focus on the content that has zero science to support it:
First, we’re met with this title. What is “Amniotic Fluid Cell Therapy”? Amniotic fluid surrounds the baby and has a few stem cells when it’s fresh and thrown into a lab culture dish right away (1), but multiple research studies have now shown that this stuff has no live and functional stem cells after it’s processed, frozen, and sold by commercial vendors to SCIA or any other clinic (2-4). So the misrepresentation begins right upfront. Or maybe they’re not talking about stem cell therapy?
Ok, here we see that the organization claims that it offers “amniotic fluid stem cell therapy”. We also see that it is “MORE EFFECTIVE” than other kinds of stem cell therapy. Hmm… First, the amniotic fluid used by SCIA and any other clinic has no living stem cells as the studies above have shown. So this isn’t a “stem cell therapy”. Second, are there clinical studies showing that amniotic fluid works better than bone marrow concentrate, a same-day stem cell therapy derived from the patient’s bone marrow? Nope. Not a single study published in the US National Libray of Medicine has compared these two therapies.
So where could this claim come from? My guess would be basic science studies comparing how amniotic fluid derived stem cells help repair tissue in comparison to adult bone marrow mesenchymal stem cells. So what do those studies show? Well here’s one that shows that for arthritis (in-vitro cartilage repair), bone marrow stem cells worked better than amniotic stem cells (5). Given that there are no references in this brochure to support this statement, there’s no way to see how the authors could back up what appears to be an inaccurate assertion.
Not even sure where to start with the statement. First, we have examined these amniotic fluid products under the microscope and they definitely do contain foreign tissue made up up microscopic chunks of dead cells. These cells have foreign HLA markers, so they can be identified as foreign by the body. This fits with what we see clinically, as patients often have huge flare-ups after these products are injected. In addition, some of these have been severe and have required hospitalization.
Second, these amniotic fluid products are only regulated with a simple, free on-line registration with the FDA (6). The agency does NO review of the product whatsoever. These manufacturers are supposed to follow communicable disease screening tests, but as we saw with the Liveyon debacle, this doesn’t always happen. In that case, many patients were made critically ill by injecting a birth tissue product that was contaminated (7). How did that happen? Again, the FDA does ZERO review of these products before they are sold to clinics.
Finally, the term “Safe and Effective” is incredibly misleading here. Again, the FDA does no review of these products for safety or efficacy. The phrase “Safe and Effective” is regulated by FDA (8) and applies to drugs or cell products (HCT/P’s) that have undergone it’s rigorous 3 phase clinical trials and review process. This is called a 351 HCT/P product which is dramatically different than the free on-line tissue registration with no review for amniotic fluid products. Hence, using the term here is not only inaccurate but seems to be to lead the reader to conclude that the amniotic product used by SCIA has been through this same arduous, multi-year, FDA approval process. Or that FDA has reviewed and cleared the product before it is sold, which is not the case.
This statement says that the amniotic fluid injection used by SCIA contain “cytokinins” and natural growth factors which result in “RELIEF & REPAIR”. In addition, these can’t be added to adult stem cells. First, I think the term “cytokinins” which is used throughout the brochure is yet another spelling error. Why? Cytokinins are plant-based messenger molecules (9)! I think the author meant to write the term “cytokines” which are small proteins that act as cell messengers and found in mammalian cells like humans (10).
Next, we hear that “cytokinins” and natural growth factors result in “RELIEF & REPAIR!” The problem with this statement is that to support it, we would need extensive publications of human clinical trials that were published in medical journals that commercially available amniotic fluid injections resulted in “RELIEF & REPAIR!” Then these studies would need to tie those results back to the cytokine and growth factor profile or levels. In fact, these studies are VERY limited.
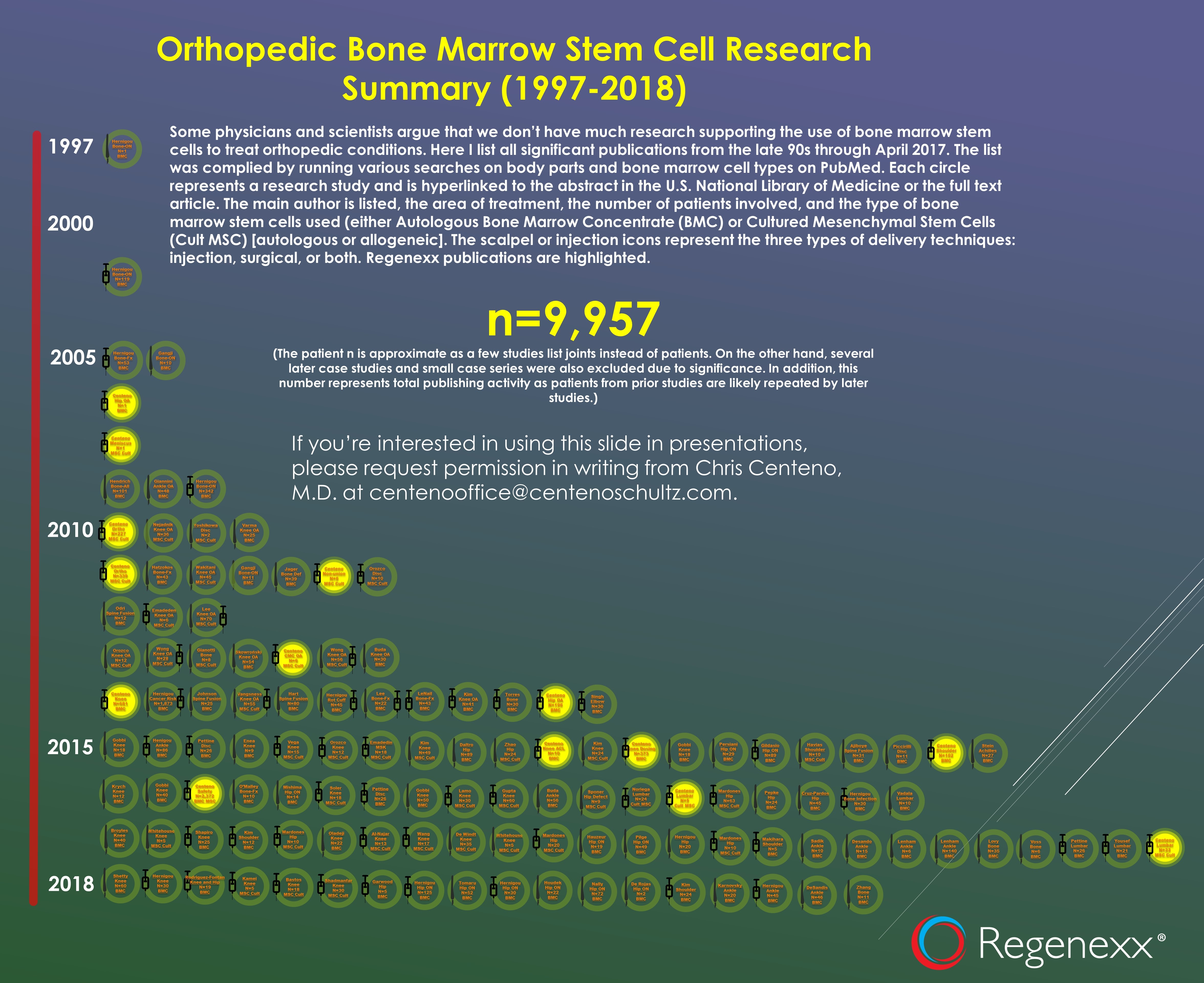
To date, there is only one pilot study published in the US Library of Medicine with 6 patients who had knee osteoarthritis (11). While there was some relief at one year, there was no evidence for repair. There is also a single case series of 44 patients who had plantar fascitis or Achilles tendinosis with only 12 weeks of follow-up that demonstrated improvement in pain but no evidence of repair (12). That’s all I can find as of this morning, a sum total of 50 patients with musculoskeletal pain who have been treated with amniotic fluid injections and who had their results reported. How does that compare, for example, to the literature showing that stem cells derived from bone marrow help musculoskeletal pain? As of last year, there were 9,957 patients who had their results reported for bone marrow derived cells and that data goes back to the 90s. That infographic is below (click to see the full document with links to studies):
How about the concept that cytokines or growth factors in the products are responsible for these effects? While there are cytokines and growth factors in these products, we have no data that these are responsible for any of the effects of these products. Nothing. How about the idea that cytokines and growth factors can’t be added to bone marrow stem cells? Since bone marrow stem cells are delivered live, they produce cytokines and growth factors (13). Obviously, since amniotic fluid stem cells are delivered dead, that doesn’t happen.
The upshot? I could go on here as there are many more problematic statements in this document. However, I think you get the big picture here. What’s in this patient brochure isn’t accurate. It’s a sales job to try and convince patients that they’re getting something they’re not actually receiving. I think they call that bait and switch.
_____________________________________________
References:
(1) Srivastava, M., Ahlawat, N. & Srivastava, A. Amniotic Fluid Stem Cells: A New Era in Regenerative Medicine. J Obstet Gynecol India (2018) 68: 15. https://doi.org/10.1007/s13224-017-1034-z
(2) Dustin R. Berger, Nicolette F. Lyons, and Neven J. Steinmetz. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(3) Liliya Becktell, Andrea Matuska, PhD, Stephanie Hon, DVM, Michelle L. Delco, DVM, PhD, Brian J. Cole, MD, Lisa A. Fortier, DVM, PhD. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Panero, A. J., Hirahara, A. M., Andersen, W. J., Rothenberg, J., & Fierro, F. (2019). Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(5) Kolambkar, Y.M., Peister, A., Soker, S. et al. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Hist (2007) 38: 405. https://doi.org/10.1007/s10735-007-9118-1
(6) US Food and Drug Administration. Tissue Establishment Registration. Accessed 7/21/19. https://www.fda.gov/vaccines-blood-biologics/biologics-establishment-registration/tissue-establishment-registration
(7) Centers for Disease Control. Outbreak and Patient Notifications. Bacterial infections after use of stem cell products – January 28, 2019. https://www.cdc.gov/mmwr/volumes/67/wr/mm6750a5.htm
(8) US Food and Drug Administration. The FDA’s Drug Review Process: Ensuring Drugs Are Safe and Effective. Accessed 7/21/19. https://www.fda.gov/drugs/drug-information-consumers/fdas-drug-review-process-ensuring-drugs-are-safe-and-effective
(9) Wikipedia entry on “Cytokinins”. Accessed 7/21/19. https://en.wikipedia.org/wiki/Cytokinin
(10) Wikipedia entry on “Cytokine”. Accessed 7/21/19. https://en.wikipedia.org/wiki/Cytokine
(11) Vines J, Aliprantis A, Gomoll A, Farr J. Cryopreserved Amniotic Suspension for the Treatment of Knee Osteoarthritis. J Knee Surg 2016; 29(06): 443-450. DOI: 10.1055/s-0035-1569481
(12) Werber B. Amniotic Tissues for the Treatment of Chronic Plantar Fasciosis and Achilles Tendinosis. J Sports Med (Hindawi Publ Corp). 2015;2015:219896. doi:10.1155/2015/219896
(13) Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi:10.1002/sctm.17-0129

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.