Stemell: Another Umbilical Cord “Stem Cell” Therapy Bites the Dust
If you read this blog, you know that we have many umbilical cord “stem cell” clinics out there that claim to be delivering live stem cells but in reality are delivering dead tissue. The FDA has been slowly but surely sending out Warning Letters to the companies that supply these clinics and yesterday another one called “Stemell” got a letter. However, how many are still out there claiming that they sell “stem cell” products? I was surprised.
Dead Stem Cells?
We and others have tested these birth tissue products from umbilical cord and amniotic tissue ad nauseam (1-3). We now have 3 university labs plus our facility that have found the same thing, despite being sold as amniotic or umbilical cord “stem cell” products, they largely contain dead tissue and dead stem cells. In fact, check out the image below from a joint project between our lab and a university. This shows how middle-aged and elderly bone marrow compare for stem cell content to 5 commonly used umbilical cord “stem cell” products. The purple dots in the test mean stem cells (the bone marrow is on the right) and the all white circles mean no mesenchymal stem cells (the umbilical cord products on the left):
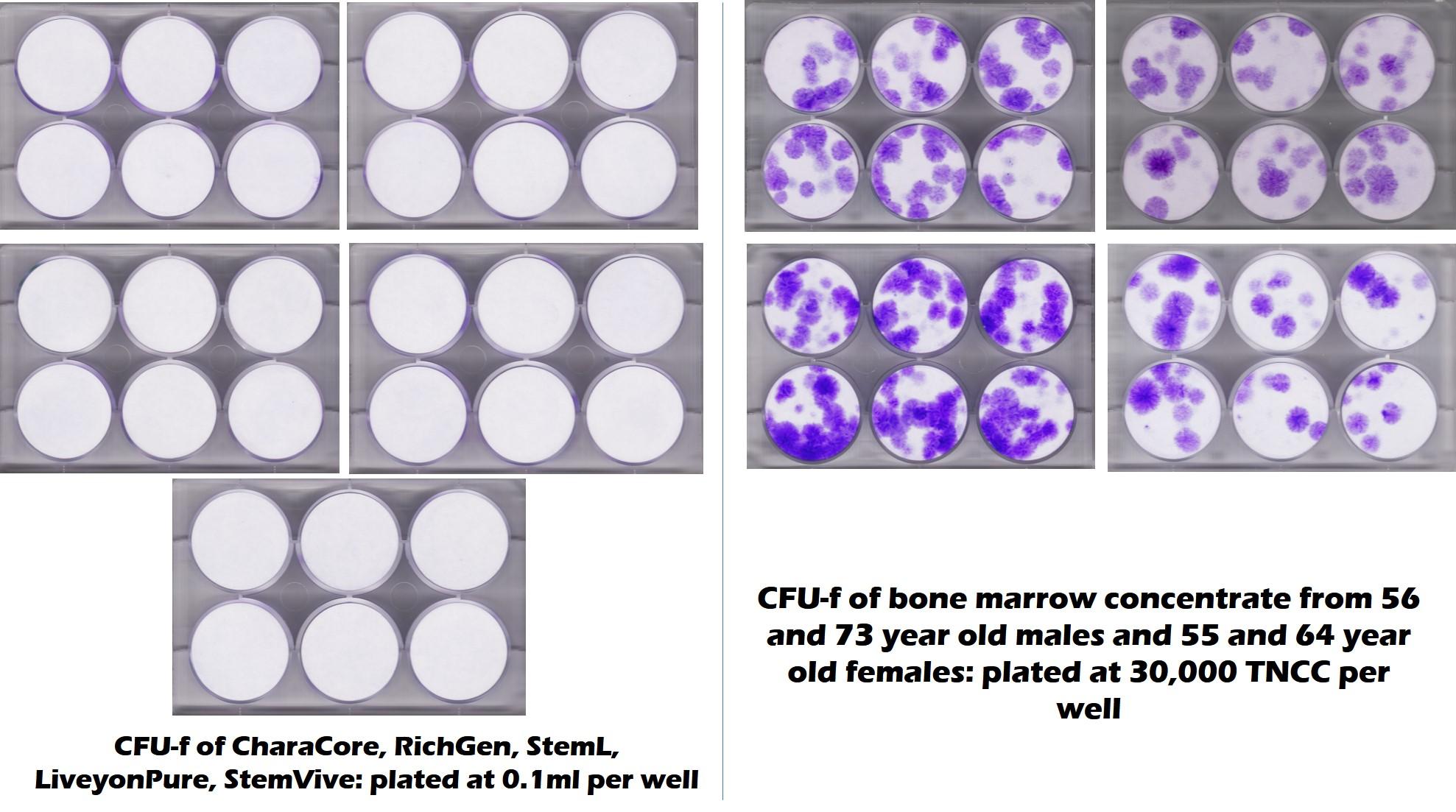
Hence, if a company claims that it sells an umbilical cord product that has live and functional mesenchymal stem cells, it’s clear at this point that this claim is not accurate. However, that’s more the Federal Trade Commission. How does that claim square with FDA guidelines?
The FDA and Umbilical Cord Vendors
The FDA has also been throwing its hat in the ring on this issue by doing what it can to shut down these companies purporting to offer live stem cells in a vial. The FDA is a “claims made” regulatory system, meaning that the agency looks at what you claim and that determines how you’re regulated. Hence, if you claim you’re selling live stem cells, tests like the one I show above aren’t critical to how you’re regulated. It’s all about the claim. Meaning if you claim to sell live cells that are actually dead, your claim is what causes what you sell to be a drug, not whether the cells are alive or dead.
Many of these companies claim to be regulated by the FDA, how does that work? These companies have always played fast and loose with FDA regulations by offering their products as a quickie 361 registered tissue despite their claims which make the products drugs that require full FDA drug approval with clinical trials (5). Watch my video below if you want to learn more about how that works:
The FDA has taken notice and begun to send out warning letters to the companies that make these products (4).
The Stemell Warning Letter
There were many chiropractic clinics in the mid-west offering what they claimed were umbilical cord derived mesenchymal stem cells that were being sold to them by a company called “Stemell”. This is one of the products we and a university tested above, clearly showing that there were no mesenchymal stem cells in the product. Yesterday the FDA issued a Warning Letter to the company that not only accuses it of marketing an unapproved drug product but also sterility and processing violations.
This is now one of a series of letters that makes it crystal clear that any of these companies that are still selling products that claim to have live stem cells are not compliant. This is the key statement from this most recent letter:
“In addition, your products fail to meet other criteria set forth in 21 CFR 1271.10(a). The umbilical cord blood product, StemL UCB-Plus™, fails to meet 21 CFR 1271.10(a)(4). This product, manufactured from donated umbilical cord blood, is dependent on the metabolic activity of living cells for its primary function and is not for autologous use, allogeneic use in a first-degree or second-degree blood relative, or reproductive use. The umbilical cord product, SternL UCT-Plus™, fails to meet the minimal manipulation criterion set forth in 21 CFR 1271.10(a)(l) and defined for structural tissue in 21 CFR 1271.3(f)(1). The product does not meet this criterion because your processing alters the original relevant characteristics of the umbilical cord related to its utility for reconstruction, repair, or replacement.”
While that’s is some dense regulatory speak, here’s what it all means:
- You claim that you’re selling live cells, which makes your product a drug that requires clinical trials and actual FDA approval-you never did that
- Your processing of the tissue alters what it was meant to do in the body, hence that another reason your product is a drug
So that begs the question, as of this morning, since the FDA has clearly stated in multiple Warning Letters that claiming to sell live umbilical cord stem cells without extensive clinical trials and approval is not legal, who else out there is claiming to sell live stem cells? Let’s dig in.
Stemell Makes a Uturn on their Website
The image below is what used to greet you on the Stemell website:
The phrase “stem cell” is right upfront. Now that term has been stripped from their website:
Predictive Biotech
Predictive Biotech is a company I have been following for years, mostly because of their bold claims of selling a mesenchymal stem cell product. I would have assumed that after the first letter from FDA came out months ago that they would have cleaned up their website. Certainly after yesterday’s letter? Nope. As you can see below, they’re still claiming that they’re selling stem cells:
“A powerhouse of untapped potential
Wharton’s jelly is a gelatinous substance in the umbilical cord that provides cushioning and support to the umbilical vein and arteries. Also known to be a rich source of cytokines, growth factors, proteins and mesenchymal stem cells. The unique characteristics associated with Wharton’s jelly have given rise to a wave of new clinical trials to understand its attributes and functions. After extensive research, Predictive believes Wharton’s jelly is unparalleled in its potential in regenerative medicine, perhaps offering a way to better the regenerative process for all people.”
Note that last column in the table above is for their product called CoreCyte which is made from Wharton’s Jelly. Note that it has a checkmark by the row which states, “mesenchymal stem cells”.
Recharg
This is a brand new one. But they’re still claiming (indirectly here) that they’re selling a “stem cell” product:
“…our ReCHaRG product contains a mixed population of cells (hemopoietic stem cells and mesenchymal stem cells)..”
Chara Biologics
We tested this company’s umbilical cord product (shown above) and found no living mesenchymal stem cells, However, the company that still claims on its website (now indirectly) to be selling a stem cell product:
They have made a few changes from the last time I visited the site, but the phrase “stem cells” is still there…
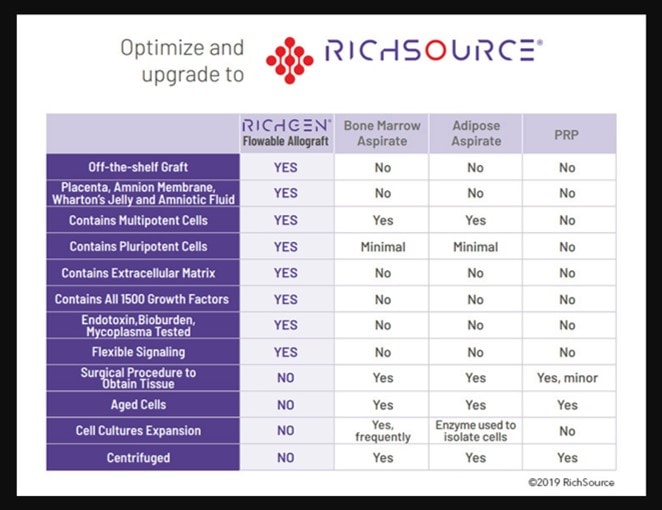
RichSource Stem Cells
We also tested this company’s product (RichGen) and found no living mesenchymal stem cells. This company is also still advertising live cells:
Note that the first column in the table above states that the “RichGen” product contains multipotent and pluripotent cells that are capable of “flexible signaling”. This is a roundabout way to say live stem cells.
We received a letter from RichSource’s attorney who claimed that RichGen is a properly registered 361 tissue product and not a 351 drug. I then sent a request to the FDA TRIP program to get a ruling on that and this is what I received:
“Dear Dr. Centeno,
Thank you for your inquiry. The Center for Biologics Evaluation and Research (CBER) is one of seven centers within the Food and Drug Administration (FDA). CBER is responsible for the regulation of biologically-derived products, including blood intended for transfusion, blood components and derivatives, vaccines and allergenic extracts, human cells, tissues, and cellular and tissue-based products (HCT/Ps), gene therapy and xenotransplantation products.
Your inquiry was forwarded within CBER to the Office of Tissues and Advanced Therapies (OTAT) to assist in response preparation, which is included below.
Thank you for contacting the U.S. Food and Drug Administration.
Human cells or tissue intended for implantation, transplantation, infusion, or transfer into a human recipient are regulated by CBER as human cells, tissues, and cellular and tissue-based products (HCT/P). FDA has implemented a risk-based approach to the regulation of HCT/Ps. Under the authority of section 361 of the Public Health Service (PHS) Act, FDA established regulations for all HCT/Ps to prevent the introduction, transmission, and spread of communicable diseases. These regulations can be found in Title 21 of the Code of Federal Regulations (CFR) Part 1271:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271.
HCT/Ps that meet all the criteria in 21 CFR 1271.10(a), are regulated solely under section 361 and 21 CFR part 1271, and no pre-market review (application to FDA) is required. To satisfy these criteria, an HCT/P must be: minimally manipulated (relates to the nature and degree of processing); intended for homologous use only (the product performs the same basic function in the donor as in the recipient); not combined with another article (with some limited exceptions); and the HCT/P does not have a systemic effect on the recipient and is not dependent upon the metabolic activity of living cells for its primary function, or if it does, the HCT/P is intended for autologous use or use by a first- or second-degree blood relative.
You inquired about “umbilical cord ‘stem cell’ products.” As a general matter, a product marketed for “patient improved regeneration” and comprised of placenta, amniotic membrane, Wharton’s Jelly and amniotic fluid would not appear to meet all the criteria in 21 CFR 1271.10(a). For establishments that manufacture an HCT/P that does not meet the criteria set out in 21 CFR 1271.10(a), and that do not qualify for any of the exceptions in 1271.15, the HCT/P will be regulated as a drug, device, and/or biological product under the Federal Food Drug & Cosmetic Act (FD&C Act) and/or section 351 of the PHS Act, and applicable regulations.
To lawfully market a drug that is also a biological product, a valid biologics license must be in effect [42 U.S.C. 262(a)]. Such licenses are issued only after a demonstration that the product is safe, pure, and potent. While in the development stage, such products may be distributed for clinical use in humans only if the sponsor has an investigational new drug (IND) application in effect as specified by FDA regulations [21 U.S.C. 355(i); 42 U.S.C. 262(a)(3); 21 CFR Part 312].
For a list of approved cellular and gene therapy products, please see FDA’s website at https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
Please note that registration with FDA in the Human Cell and Tissue Establishment Registration (HCTERS) database on CBER’s website means that an establishment has notified FDA of its business address and the HCT/Ps the establishment manufactures. As provided in 21 CFR 1271.27(b), FDA acceptance of an establishment registration and HCT/P listing form does not constitute a determination that an establishment is in compliance with applicable rules and regulations or that the HCT/P is licensed or approved by FDA.
I hope this information is helpful.
Sincerely,
Stacey Rivette
Consumer Safety Officer”
However, we sent that letter to RichSource who sent this response back:
“We have reviewed your letter of September 20, 2019, carefully. There is, as you know, nothing in the FDA Jetter that supports your clients’ assertions. My client’s product is properly distributed pursuant to Section 361 of the Public Health Service Act and CFR section 1271.lO(a) as it comes from a single donor, is minimally manipulated, is intended for homologous use and meets the other requirements.”
So is RichGen a drug without approval or a properly registered 361 tissue product? That’s up to you to decide after reading all of this stuff.
StemVive-Utah Cord Bank
We also tested this product and also found no live and viable mesenchymal stem cells. Interestingly, this page is also still claiming to sell a stem cell product:
In addition, equally problematic, it also claims that StemVive is “safe and effective” a huge regulatory “no-no” that FDA heavily polices. This also states that the product is made from Wharton’s jelly and that this is an “excellent source of mesenchymal stem cells (MSCs)…”
Liveyon Pure
As I noted above, Liveyon (and it’s supplier) was one of the first companies to receive letters from the FDA. I guess the good news is that they seem to have removed the term “mesenchymal stem cell” from their main website. However, the site still has collateral making some stem cell claims, which according to the letter sent to the company isn’t kosher. They also still have language in a white paper on their website that refers to stem cells:
“There is a thirty-year history of CB therapies. Thus, more than 30,000 CB transplants have been performed to treat hematopoietic malignancies, marrow failure, immunodeficiency disorders, and inborn metabolic diseases (2). Most recipients (about 70%) did not have an HLA-matched sibling donor. Cord blood cells can have “reduced alloreactivity”; this permits transplant without the need to perfectly match tissues (3). In addition to hematopoietic stem cells (HSC), CB contains a variety of other beneficial cell types. These include endothelial progenitor cells (EPC) and mesenchymal stem cell (MSC). EPCs can help form new vasculature. MSCs can help form bone, muscle, cartilage and release a host of reparative growth factors and proteins. There are other types of beneficial cells in CB that are under current study (4, 5).”
The upshot? Despite the many FDA warning letters, we still have many companies that are claiming to sell live stem cells. Given the trends, in my opinion, this seems ill-advised.
______________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(3) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(4) US Food and Drug Administration. “FDA sends warning to company for marketing dangerous unapproved stem cell products that put patients at risk and puts other stem cell firms, providers on notice” 12 August 2019, https://www.fda.gov/news-events/press-announcements/fda-sends-warning-company-marketing-dangerous-unapproved-stem-cell-products-put-patients-risk-and .
(5) US Food and Drug Administration. “Tissue Establishment Registration” 11 August 2019, https://www.fda.gov/vaccines-blood-biologics/biologics-establishment-registration/tissue-establishment-registration.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.