The “Save the Cord” Ripoff: The Insurance Policy for Very, Very, Very Rare Diseases

Expecting parents often contact me to ask my opinion about saving their baby’s umbilical cord tissue. The companies that sell this service are aggressive, and this morning, I’d like to share one such sales pitch because IMHO it’s so far off from reality that it’s nutty. Let’s dive in.
Sales 101
My wife recently bought a new dining room table. When I asked when we would get it, the sales person told her it would take a few weeks because it had to be made overseas. We’re now six months in and no table. Why? Once she spoke to the store manager after the sale, it turns out that the salesperson that sold her the table fibbed a bit. The table was really 6-9 months out from being in stock. The point of sharing this is that what salespeople say to make a sale and reality are often two different things.
Cord Blood and Tissue
Cord blood and Wharton’s Jelly both contain stem cells. If you take these tissues from an OB ward and plate these cells, you can get stem cells to grow. However, commercial companies selling these tissues as containing live and functional stem cells not only produced dead cell products but also violated FDA drug laws. In between these two extremes is the umbilical cord tissue industry, which likely does a better job of preserving the cells in these tissues. However, when it comes to selling new parents this product, it fails to deliver anything valuable. Let’s dig deeper to understand why that’s the case.
Save the Cord!
About 20 years ago, we began to see companies that for a fee, would save your baby’s umbilical cord cells for future use. The sales pitch was simple: save these cells now so that if your baby gets sick, they can use the stem cells in this tissue to help heal their body. This has become big business, with almost every OB’s office in the country (for a cut of the action) hawking this service to expectant parents.
A Colleague and a Sales Pitch
This past week, a physician colleague called me about his daughter wanting to save her baby’s cord stem cells. She was pitched by a company called “Vital Cells.” She was told that these stem cells had many different clinical applications, from heart disease to neurologic disease to orthopedics, and, as such, would be readily available for her child’s use. The company has a whole page of videos where statements like this are made:
“We have partnerships with Regenerative medicine physicians nationwide. Meaning we can connect you with the top physicians to provide further information and education about potential uses for your stem cells.”
“You can easily have access to these cells today and throughout your child’s life.”
From talking to his daughter and from watching these videos, some things become clear:
- Vital Cells is saying that you can use your child’s cells anytime you want to for any condition.
- Vital Cells will send these cells to a physician who can treat your child.
The Problem with the Sales Pitch
To understand why this sales pitch doesn’t hold water, first, let’s look at the Vital Cells website (different from the educational videos which are stored off the website):
“Many patients are already benefiting from regenerative medicine therapies using their own cells to help heal their body. There are various stem cell therapy offerings within regenerative medicine. At VitalCells, the focus is on the safest and most accurate offerings – your cells, with your own DNA, with accurate live stem cell and viability counts. With CellMaxxTM, VitalCells proprietary technology that COLLECTS, PROCESSES and GROWS cells making them AVAILABLE for your child throughout their lifetime. By storing your child’s newborn cells, you have now ensured they have potentially billions of their own youngest, personal stem cells beyond their childhood. This makes storing your newborns cells a lifelong decision.”
While this paragraph is a bit ambiguous, it sounds like you could potentially use these cells now to heal your child.
What can you use these cells for? The company has an “FDA Approved Uses” page:
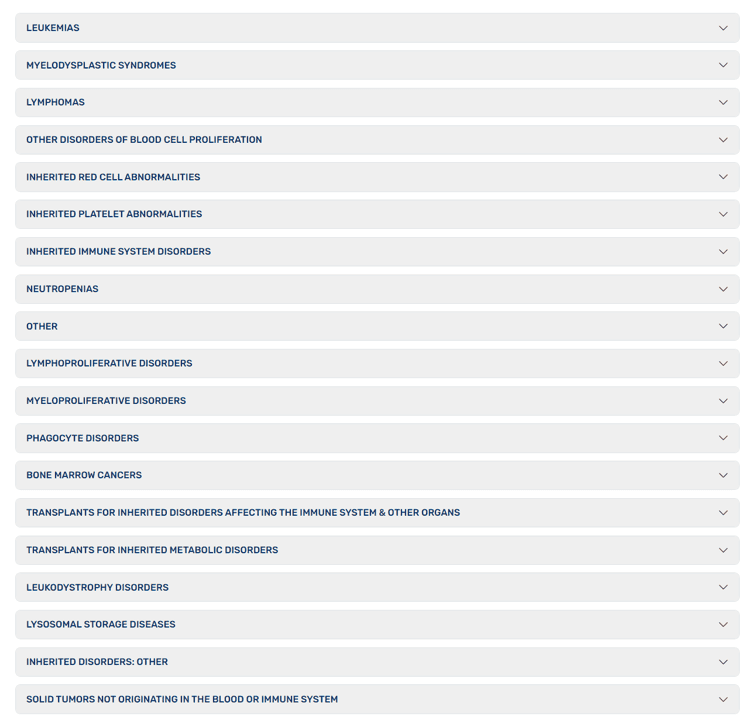
This isn’t a list of common problems adults or children suffer, but very rare disorders. Let’s explore how likely your child is to have one of these problems. For example, tragically, in 2021, there were 42,939 child traffic fatalities. Compare that to about 3,000 cases of Acute Lymphoblastic Leukemia (ALL) per year, which is the most common childhood cancer. How about transplants? That’s about 1,900 kids a year. How about “Bone Marrow Cancer” (leukemia), which causes about 3-4,000 cases annually. Hence, the chances of your kid getting any of these problems for which the FDA has approved the use of cord-derived stem cells are very, very, very small.
Cord Blood FDA Approval History
How was this FDA-approved uses list created? Way back when, I was involved in one of the first battles to define how autologous culture-expanded stem cells would be regulated. As a result of a FOIA request, we got the whole 20,000-page history of the existing FDA 1271 stem cell regulations. In reading all of that, I learned that in the late 90s, the FDA had decided to turn cells into drugs requiring full clinical trials for FDA approval. This was a massive change and there was plenty of complaining from industry experts. One product in the pipeline (Carticell) was fast-tracked as a compromise, and the other big compromise was with the cord blood banking industry. The list you see above is the known possible uses for cord blood stem cells as of the late 90s, which was grandfathered in and frozen in time. Hence, after the late 90s, for problems not on the list, like using your child’s cord stem cells to regrow knee cartilage, a full FDA drug approval would need to be in place, requiring a decade of clinical trials and hundreds of millions of dollars.
An Insurance Policy that Can Never Pay Off
So why have Vital Cells mentioned that you can use these cells whenever you want? Why are they being coy about how that works? IMHO because cord blood has always had a problem. It’s like buying a health insurance policy that only covers super rare diseases but doesn’t cover you for that back surgery or blood pressure medicine you might need. Hence, if the company selling this service can convince parents that these cells can be used for many common conditions, it’s easier to make a sale.
Can Cord Stem Cells Be Used for Whatever You Want?
The short answer is a resounding NO. The FDA has made it clear in numerous Untitled and Warning Letters that it regulates umbilical cord stem cells as a drug that requires FDA approval and regulation (see letter 1, letter 2, letter 3, letter 4, letter 5, and letter 6). Based on the FDA’s statements and actions, there is no doubt that any use of these cells to cure cardiac, neurologic, or orthopedic disease is not permitted until full FDA drug approval is granted for that clinical application.
Let’s explore what that would look like for a child born today who might want to use these cells in 20-30 years. First, will the cord bank be in business? This is a serious concern as these companies are not Microsoft or IBM, meaning they are small businesses typically with fewer than 100 employees.
Second, will you be able to use these cells? To understand that, you need to learn about FDA cellular drug law.
These umbilical cord banks DO NOT have FDA approval. Instead, the cord tissue bank filled out a 45-minute 361 registration form. In addition, the act of culture expanding the stem cells (growing more), which is what Vital Cells advertises, creates an unapproved drug product that can’t be used in humans without full FDA drug approval and clinical trials. Even advertising cord blood or Wharton’s Jelly cells that are not cultured as a product that can be used for X disease (not on the list above for cord blood) again means that full FDA drug approval is required. To learn more about this 361 tissue versus 351 drug product distinction, watch my video below:
What does all of this mean? The following:
- Full FDA clinical trials and approval are needed for each clinical use (e.g., damaged knee cartilage). Since this takes about a decade and costs a few hundred million dollars for each clinical indication, very few, if any, of these for autologous umbilical cord stem cells will exist in even 20 years.
- Each drug product above will be tied to a specific manufacturer, process, and release criteria. This means the company that spends 300 million dollars to get a specific clinical indication approved will be the only product that can be used. In particular, unless that company is Vital Cells, your chance of riding coattails on that approval is almost nil.
The ACT/Vital Cells Link
An expert in cord blood banking, Fran Verter, Ph.D., pointed out on Linkedin that Vital Cells has the same address as a company I previously covered called American Cell Technology. In fact, the Vital Cells website says:

A LinkedIn search turned up only two employees for VitalCells. One is the salesperson who spoke to my colleague’s daughter and a COO. In fact, “Keven T.F.” is listed as the COO of both VitalCells and ACT. Bizarrely, Keven T.F. is actually Keven T. Ferber. Mr. Ferber is all over social media promoting multiple medical indications for which his 361 registered cells can be used. IMHO, and according to multiple FDA Warning and Untitled Letters, that promotion makes those cells an unapproved drug product.
American Cell Technology on LinkedIn has 10 employees. This includes Elena Rusyn, the Ukrainian doctor and ACT CSO, whom I have previously written about. In 2020, Elena, who lives in California, was trying to get a US medical license. A current California medical license look-up shows no medical license for this individual.
Why was ACT important? Because it seemed to be the current reincarnation of US Stem Cell, a company that went down in flames about a decade ago. So VitalCells is the third incarnation of US Stem Cell.
It should also be noted that VitalCells is not an FDA-registered tissue bank, but ACT has a 361 registration, as described above.
What About Shipping These Cells Overseas?
In my experience, salespeople for these umbilical cord banks often say that if the cells can’t be used here, they can be sent overseas and used there. Is this true? Maybe. The 361-tissue bank facility would have to produce the cells as an unfinished drug product for that to work. That means that the cells are made using a full c-GMP cell drug set-up, which just doesn’t exist in these cord tissue banks. The difference between the GLP standard many of these labs follow and the drug manufacturing process is MASSIVE. The problem is that cGMP drug manufacturing adds thousands to the cost of each cell drug dose.
Could the cells produced using the lesser standard qualify for export as an unfinished drug product? Nope, not legally. Meaning these cells could be confiscated by federal authorities as an illegal drug product. Could it fly under the radar? Yep, but do you want to take that risk?
Does Any of This Make Common Sense?
Given that hundreds of cell therapy and regenerative medicine products are currently winding their way through FDA and other drug approval processes in other countries, why would you want to save your child’s cells when your time horizon for therapy is decades? Meaning 20-30 years from now, there will be dozens of FDA-approved and insurance-covered cell therapies from many sources for many different problems with better clinical data than exists today for using autologous umbilical cord cells. Thirty years from now, if you were to go to a doctor and say that you have banked cells at company X (assuming that company still exists), they will laugh and steer you to one of the cell therapies that are FDA-approved and covered by insurance.
The upshot? Umbilical cord banking is the ultimate insurance policy that will never pay off. IMHO, this is a silly idea, and the sales pitch these companies bombard young parents with doesn’t hold water. If I were having a new baby, I’d save that money and put it in a good 529 college fund instead!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
