The Alien Abduction Model of DDD: Why Cellular Drug Trial Results Have Been Underwhelming

Credit Shutterstock
Let’s say that you’re sleeping one night and get awoken by an alien in your bedroom who takes you back to the mothership for experiments on your low back. That’s pretty much what we’ve been doing with animal degenerative disc disease research for decades. It’s this artificial DDD model that has caused many cell therapy drug trials to fail or underperform. Hence, let’s get into what’s happening and how this problem can be solved.
The Alien Abduction Model of DDD
In the cell therapy industry of companies pursuing FDA approval as a drug, billions of dollars of investor money have been flushed down the drain. Why? Because these companies rely on animal models that show miraculous results but then can’t be replicated in humans. Nowhere is this more obvious than in companies seeking cellular drug approval to treat one of modern man’s most common afflictions, lumbar degenerative disc disease (DDD).
When you see an animal study on DDD, there’s always a big problem. Rats, rabbits, and sheep are quadrupeds. They do not generally get DDD in their lifetimes. Hence, if you want to experiment on their vastly different, little low back discs, you need an experimental way to create DDD. How does that work?
To create DDD where none usually exists, as a researcher, you take a needle that’s almost as large as the disc itself and you suck out the nucleus pulposis (NP) or the inside gel part of the disc. After doing that, in a few weeks, you get something that looks like DDD but is not at all like human DDD. In fact, the animal DDD models are much more like being abducted by aliens who stick a probe into your disc and suck out the NP and then return you back to your bedroom (after erasing your memories).
The reason I call this the alien abduction model is to demonstrate how this artificially created DDD is ridiculous. Despite that silliness, this DDD animal model is used by many biotech companies to raise millions of dollars. Let’s explore some of that research today.
The Big Picture of DDD Cell Therapy Research
Many biotechs have tried to move from the alien abduction model of DDD quickly into human trials. Why? Because the alien abduction model of DDD is easy to treat. Injecting allogeneic stem or other cells (usually disc or birth tissue cells) into rat or rabbit discs usually causes complete healing and reconstitution of the “degenerated” disc. They use these amazing results to raise investor money, but then find out the hard way that real human DDD is very different as the FDA clinical trial results are quite different.
How is real human DDD very different than the animal model? Here are a few disconnects:
- Real human DDD takes place over years or decades. It doesn’t happen in one night on a spaceship.
- Humans are bipeds that constantly load their discs when seated or upright, animals are quadrupeds that use their discs as a bending linkage between vertebrae.
- Many humans with DDD are usually older with metabolic syndrome with high levels of pro-inflammatory cytokines. Animals with alien abduction DDD are younger and fully healthy, usually at the prime of their short lives.
Lipstick on a Data Pig
Let’s say you’re a CEO or CMO of a pharma company perusing a cell therapy for DDD. Because you raised millions of dollars using the alien abduction model of DDD, your human clinical trial results come back looking very different than they looked in a rat or rabbit. You now have a very big problem that you need to solve with creative statistics.
One of the ways that companies have tried to hide lackluster trial results is by using a little trick called subgroup analysis. What’s that? Let’s say you have two groups, one treated with a disc cell injection and another with saline. You run the stats and don’t see much difference in the mean outcome score between the groups. However, you know that reporting a negative trial result will waste tens of millions invested in the clinical trial. Have no fear, “subgroup analysis” will usually solve your problem!
Subgroup analysis means that you try to find one portion of the treated patients who seemed to do better and then focus only on that group. Hence, you now state that 4 in 10 patients responded well, therefore the product works! Does it work well? Nope. However, if you play the subgroup game well, you may still sneak it by an FDA drug approval committee.
The Companies that Have Tried
This is not an exhaustive list, but just a few of the companies that are trying to get approvals for cell drugs to treat DDD. Here I’ll focus on how most have reported lackluster results because of their reliance on alien abduction DDD models. All began with that animal model and based everything from their outcome endpoints to their trial design on those results.
Mesoblast
The first entrant in the alien abduction DDD game was mesoblast. They have an allogenic degenerative disc disease product derived from healthy young bone marrow mesenchymal stem cells that showed miraculous results in animal models. They have also been mired in the FDA clinical trials process for almost a decade.
I blogged on these trial results in 2020. They also reported results in 2021. Here are my conclusions:
- They missed their structural imaging endpoint in 2020 (trying to show that they had improved DDD on MRI), so they focused on minute decreases in instability at that disc which are too small to measure clinically.
- Their “positive” 2020 results relied on subgroup analysis. Their 2021 reported results used the same trick, focusing on patients who had low back pain due to DDD for shorter durations.
- They only measured pain relief for 24 months. Given the immense expense of a cell therapy product, that’s not going to cut it with insurers.
- They also reported poor pain relief results in 2021, so they relied on a new FDA-approval metric called “Opioid Avoidance”. That means that patients who were using opioids used less.
Will the FDA grant approval? That remains to be seen. However, I think the company may have a hard time getting coverage from most insurers given the lackluster results.
Via Disc
Via Disc is a company that has taken a different approach than mesoblast. The company, called Vivex Biologics, uses ground-up donor disc tissue in saline. There seems to be some debate as to whether there are viable NP cells in the product.
The company published its 12-month clinical trial results in 2021. Everything you need to know about this study is in this graph I created:
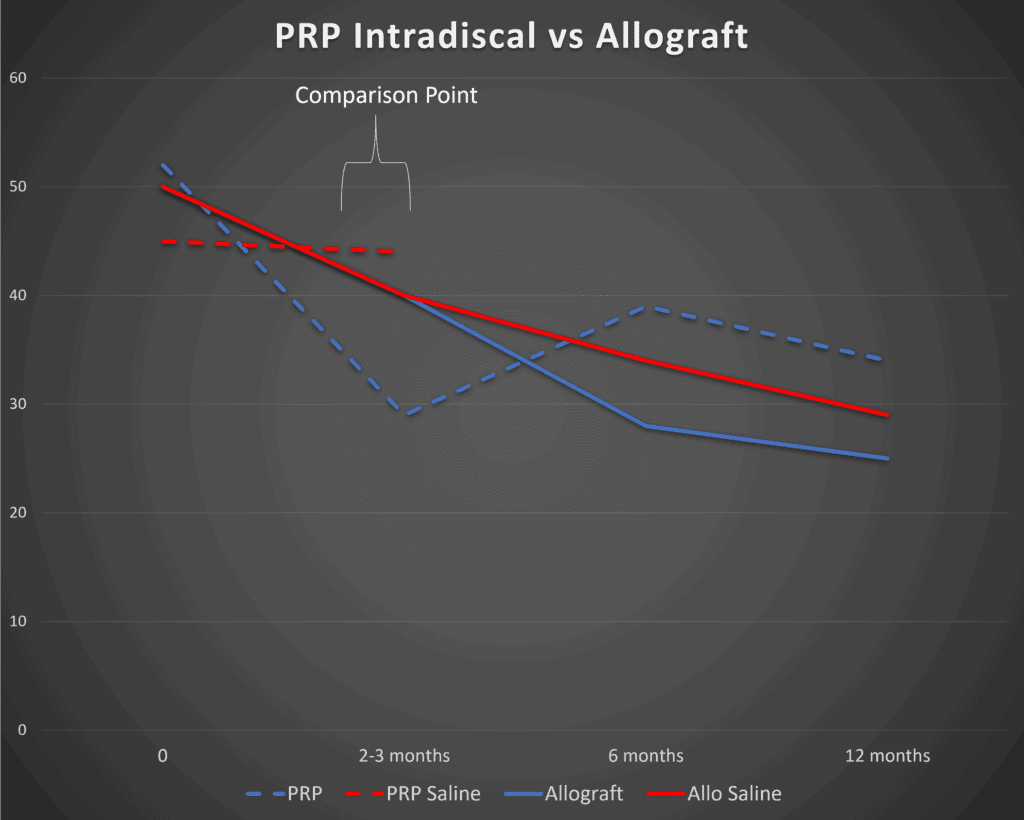
We see some serious problems. Note at the 3 months mark (marked 2-3 months here) that there is zero difference between the VIA Disc and saline injections (solid lines – Allograft and Allo Saline). A small difference between the groups doesn’t show up until 6 months. When intradiscal PRP is compared to saline (Lutz study – dashed lines – PRP and PRP Saline) we see bigger differences.
The company also relied on “subgroup analysis”. They also abandoned the concept that their product was going to show objective improvements in MRI appearance of degenerated low back discs, which they had claimed in pilot studies.
DiscGenics
DiscGenics uses culture-expanded adult disc cells combined with a delivery vehicle in a product they call “rebonuputemcel”. The company reported positive results from its early phase clinical trial (I/II) in February of 2022. Given the early stage of this company and the fact that no specifics of their data have been reported to date, it’s hard to say if this company will suffer the same fate as Mesoblast and Vivex.
Autologous Orthobiologics DDD Research
All of these companies have a serious problem in that autologous orthobiologics like platelet-rich plasma and bone marrow concentrate have a significant headstart in human research. In addition, these treatments and procedures didn’t begin in animals but evolved clinically as physicians tried to help patients. Hence, they didn’t have lofty aspirations of regrowing discs based on alien abduction animal experiments. Finally, they are far cheaper than any off-the-shelf biologic drug.
PRP
PRP is platelet-rich plasma created from the patient’s blood. Lutz et al have published a series of papers using leukocyte-rich PRP (LR-PRP) injected into the discs of DDD patients with excellent results compared to placebo saline injections (1,4). That procedure has since been optimized using higher-dose PRP (2). These findings of higher platelet concentration leading to better intradiscal injection clinical results were also echoed by Jain et al (3).
The safety of these procedures is good, with the caveat being that like all disc injections, discitis can occur (5,7). This means that the bacterial balance of the disc is upset and it becomes infected. Several studies (including one that we performed with Lutz et al) on reducing the prevalence of discitis have shown promising in-vitro results, but those have yet to be verified clinically (6).
After using this treatment for many years in our clinic, it seems to work well in real patients with disc pain or tears in the disc. As we have reported many times, the research seems to support that higher concentrations of PRP will usually lead to better clinical results. In addition, in our extensive experience in treating DDD with orthobiologics, to get the best results you need to treat the whole functional spinal unit (the irritated nerves, facet joints, atrophied muscles, and lax ligaments), not just the disc.
BMC
Bone Marrow Concentrate or BMC is the isolated stem cell containing fraction from bone marrow aspirate. The research on using BMC injected into low back discs was reported years ago and was also dependent on the number of cells delivered, meaning the patients that got a higher number of stem cells reported superior outcomes (8-10). Wolff et al also reported good results using BMC injected into lumbar discs (14). Recently Atluri et al reported excellent results on using disc BMC injections as well as treating the functional spinal unit as discussed above (11).
Culture Expanded Bone Marrow Derived Mesenchymal Stem Cells (MSCs)
Our group has been using PRP and BMC in discs for many years, more focused on using these treatments for patients with disc pain due to a tear. However, in our clinical experience, nothing will reliably treat a disc bulge unless you use culture-expanded MSCs that are hypoxic conditioned in culture.
Our experience in using MSCs in the disc was the first in the world back in 2005. Since then we’ve published two case series with MRI reports of decreased disc bulge sizes (12,13). Others have published on the use of culture-expanded bone marrow MSCs injected into the discs of DDD patients (15).
How to Fix Low Back Disc Cell Therapy Clinical Trials?
If these companies who are trying to produce off-the-shelf DDD therapies are to get both FDA approval and present a case to insurance companies, there are lessons to be learned here:
- Do not trust alien abduction animal models of DDD! Meaning the fact that you regrew a disc in a rat or rabbit means nothing when treating real human low back DDD.
- You need real-world experience in how to use your product! There are countries that permit the use of culture-expanded or other cell therapy products that require FDA approval in the United States. You need real-world experience on which patient population can be successfully treated and which patients will not respond. That gets rid of the silly subgroup analysis being used by these companies in FDA clinical trials.
- Based on the existing research, your own (autologous) cells injected into discs stick around and differentiate (16). However, there is mounting evidence that someone else’s cells (including stem cells) initiate an immune response in the host and likely are “taken out” by the cellular immune response and HLA mismatch (17,18). Hence, an autologous study is more likely to succeed.
The upshot? Let’s stop using the amazing results of nutty alien abduction models of DDD to raise money for cell therapy products! Real-world experience is needed in this field because human DDD is unique and doesn’t really exist in rats and rabbits. In addition, any drug that does get past the FDA gauntlet is going to have to outperform much cheaper and much more established autologous orthobiologics to convince United or BC/BS that it’s a cost saver.
________________________________________________
References:
(1) Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, Harrison JR, Gribbin CK, LaSalle EE, Nguyen JT, Solomon JL, Lutz GE. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM R. 2016 Jan;8(1):1-10; quiz 10. doi: 10.1016/j.pmrj.2015.08.010. Epub 2015 Aug 24. PMID: 26314234.
(2) Lutz C, Cheng J, Prysak M, Zukofsky T, Rothman R, Lutz G. Clinical outcomes following intradiscal injections of higher-concentration platelet-rich plasma in patients with chronic lumbar discogenic pain. Int Orthop. 2022 Jun;46(6):1381-1385. doi: 10.1007/s00264-022-05389-y. Epub 2022 Mar 28. PMID: 35344055; PMCID: PMC9117340.
(3) Jain D, Goyal T, Verma N, Paswan AK, Dubey RK. Intradiscal Platelet-Rich Plasma Injection for Discogenic Low Back Pain and Correlation with Platelet Concentration: A Prospective Clinical Trial. Pain Med. 2020 Nov 1;21(11):2719-2725. doi: 10.1093/pm/pnaa254. PMID: 32869064.
(4) Cheng J, Santiago KA, Nguyen JT, Solomon JL, Lutz GE. Treatment of symptomatic degenerative intervertebral discs with autologous platelet-rich plasma: follow-up at 5-9 years. Regen Med. 2019 Sep;14(9):831-840. doi: 10.2217/rme-2019-0040. Epub 2019 Aug 29. PMID: 31464577; PMCID: PMC6770415.
(5) Beatty NR, Lutz C, Boachie-Adjei K, Leynes TA, Lutz C, Lutz G. Spondylodiscitis due to Cutibacterium acnes following lumbosacral intradiscal biologic therapy: a case report. Regen Med. 2019 Sep;14(9):823-829. doi: 10.2217/rme-2019-0008. Epub 2019 Aug 19. PMID: 31423905.
(6) Prysak MH, Lutz CG, Zukofsky TA, Katz JM, Everts PA, Lutz GE. Optimizing the safety of intradiscal platelet-rich plasma: an in vitro study with Cutibacterium acnes. Regen Med. 2019 Oct;14(10):955-967. doi: 10.2217/rme-2019-0098. Epub 2019 Oct 7. PMID: 31587600.
(7) Jerome MA, Lutz C, Lutz GE. Risks of Intradiscal Orthobiologic Injections: A Review of the Literature and Case Series Presentation. Int J Spine Surg. 2021 Apr;15(s1):26-39. doi: 10.14444/8053. Epub 2021 Apr 21. PMID: 34376494; PMCID: PMC8092939.
(8) Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop. 2017 Oct;41(10):2097-2103. doi: 10.1007/s00264-017-3560-9. Epub 2017 Jul 26. PMID: 28748380.
(9) Pettine K, Suzuki R, Sand T, Murphy M. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. Int Orthop. 2016 Jan;40(1):135-40. doi: 10.1007/s00264-015-2886-4. Epub 2015 Jul 10. PMID: 26156727.
(10) Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015 Jan;33(1):146-56. doi: 10.1002/stem.1845. PMID: 25187512.
(11) Atluri S, Murphy MB, Dragella R, Herrera J, Boachie-Adjei K, Bhati S, Manocha V, Boddu N, Yerramsetty P, Syed Z, Ganjam M, Jain D, Syed Z, Grandhi N, Manchikanti L. Evaluation of the Effectiveness of Autologous Bone Marrow Mesenchymal Stem Cells in the Treatment of Chronic Low Back Pain Due to Severe Lumbar Spinal Degeneration: A 12-Month, Open-Label, Prospective Controlled Trial. Pain Physician. 2022 Mar;25(2):193-207. PMID: 35322978.
(12) Centeno C, Markle J, Dodson E, Stemper I, Williams CJ, Hyzy M, Ichim T, Freeman M. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017 Sep 22;15(1):197. doi: 10.1186/s12967-017-1300-y. PMID: 28938891; PMCID: PMC5610473.
(13) Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med. 2016 Sep 1;14(1):253. doi: 10.1186/s12967-016-1015-5. PMID: 27585696; PMCID: PMC5009698.
(14) Papadimitriou N, Hebelka H, Hingert D, Baranto A, Barreto Henriksson H, Lindahl A, Brisby H. Intradiscal Injection of Iron-Labeled Autologous Mesenchymal Stromal Cells in Patients With Chronic Low Back Pain: A Feasibility Study With 2 Years Follow-Up. Int J Spine Surg. 2021 Dec;15(6):1201-1209. doi: 10.14444/8152. PMID: 35086878.
(15) Wolff M, Shillington JM, Rathbone C, Piasecki SK, Barnes B. Injections of concentrated bone marrow aspirate as treatment for Discogenic pain: a retrospective analysis. BMC Musculoskelet Disord. 2020 Feb 28;21(1):135. doi: 10.1186/s12891-020-3126-7. PMID: 32111220; PMCID: PMC7049206.
(16) Henriksson HB, Papadimitriou N, Hingert D, Baranto A, Lindahl A, Brisby H. The Traceability of Mesenchymal Stromal Cells After Injection Into Degenerated Discs in Patients with Low Back Pain. Stem Cells Dev. 2019 Sep 1;28(17):1203-1211. doi: 10.1089/scd.2019.0074. Epub 2019 Jul 23. PMID: 31237488.
(17) Ryan AE, Lohan P, O’Flynn L, Treacy O, Chen X, Coleman C, Shaw G, Murphy M, Barry F, Griffin MD, Ritter T. Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cell transplantation. Mol Ther. 2014 Mar;22(3):655-667. doi: 10.1038/mt.2013.261. Epub 2013 Nov 1. PMID: 24184966; PMCID: PMC3944342.
(18) Rowland, A.L., Xu, J.J., Joswig, A.J. et al. In vitro MSC function is related to clinical reaction in vivo. Stem Cell Res Ther 9, 295 (2018). https://doi.org/10.1186/s13287-018-1037-4

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
