Why Is R3 Still Advertising Stem Cells for Sale?
Here we are, half a year beyond the FDA regulatory crackdown, and you would think that the scam that was umbilical cord “stem cells” was long since over. However, yesterday I got an R3 “stem cell” ad sent to me by a colleague. Let’s dig in.
The Urban Myth of Umbilical Cord Stem Cells
If you read this blog, you know that anyone claiming to sell umbilical cord “stem cells” in a vial here in the US is not selling live and functional stem cells. Our research paper that was published in the Americal Journal of Sports Medicine clearly demonstrated that none of the companies claiming to be selling vials of stem cells actually had any live and functional stem cells (1). Hence, anyone selling this product and making this claim is IMHO almost certainly selling fairy dust.
What Is R3?
R3 is a marketing company run by David Greene, M.D. David used to be an orthopedic surgeon but lost his medical license. There’s an extensive deep dive into R3 at the link.
The Recent Ad
Last night I was sent this ad by a colleague:

This came off of Facebook and was served up last night. It’s got all sorts of issues. The first IMHO is the claim that R3 is selling stem cells in a vial, which based on our research and that published by others is false (1-4). The second claim that’s not accurate IMHO is that there 10 million live “stem cells” in these vials. These numbers when used by umbilical cord tissue resellers refer to the total number of cells and not the number of dead or dying stem cells. That dead and dying stem cell number would be a small fraction of the total number. Finally, we have no published data that supports that the umbilical cord product being used by R3 relieves pain.
R3’s Website
One of the hallmarks of the post-FDA Crackdown world is that the resellers of umbilical cord tissue have mostly scrubbed their websites clean of stem cell claims and have pushed these false claims to the sales reps that interact with physicians. This reduces their footprint for drawing the ire of the FDA. So what did R3 do?
This is their website this morning. Did they clean it up? Not really. While the main messaging has now changed to regenerative medicine, it’s still claiming that its product contains stem cells.
What Type of Clinics are Still Offering this Stuff?
You would think that every reputable physician out there has heard that:
- Umbilical cord products like the ones that R3 is selling contain no live and functional stem cells
- The FDA doesn’t allow physicians to use or R3 to sell these products advertised this way
Hence, who would take this risk? To answer that question I picked four clinics from the 35 that R3 lists on their website and this is what I found:
Colorado
This is the clinic:

This is listed online as the office of a nurse practitioner. No medical specialist is listed at this site.
Tennesee
It was no surprise to see IMAC Regeneration Center listed in Tennessee. I’ve blogged on these guys before. This is a clinic chain run and owned primarily by chiropractors that that trades on the NASDAQ. Here’s the company’s stock price during the ridiculous stock market inflation of the last few years:
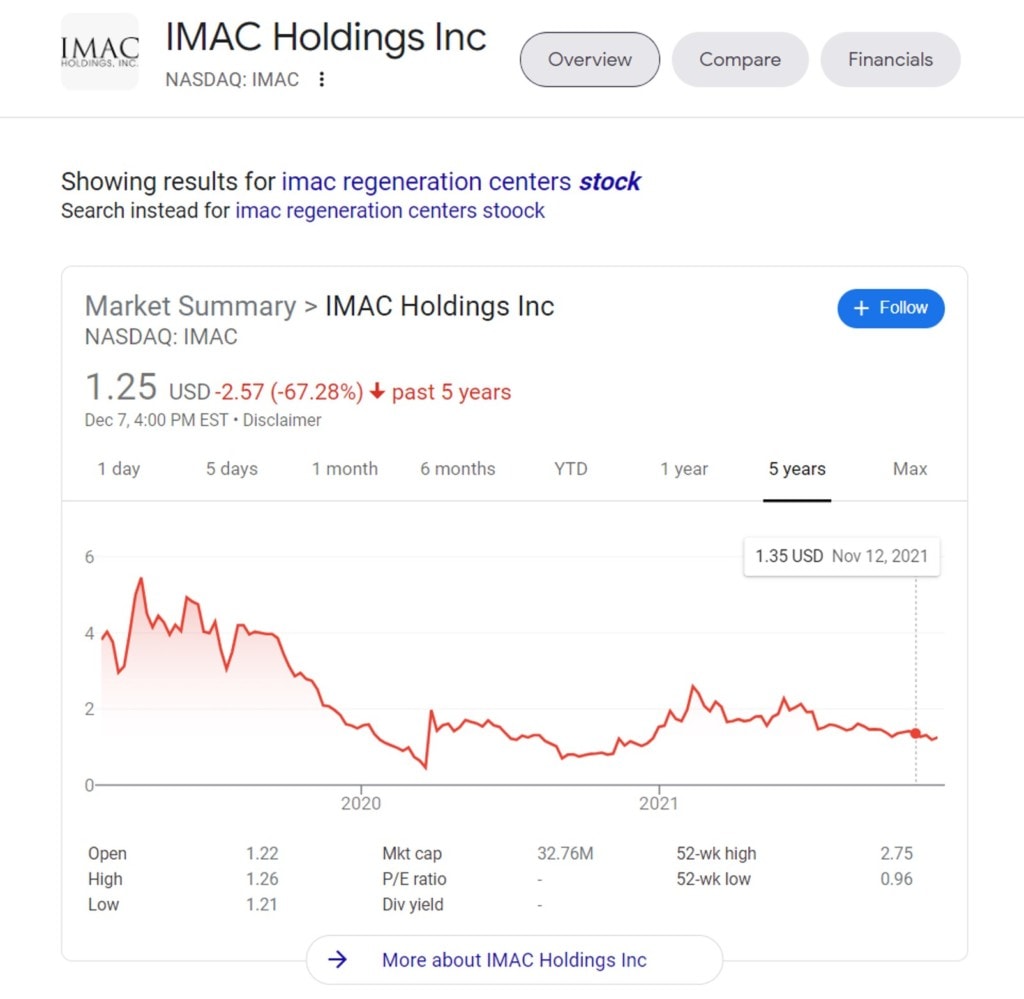
During this time the NASDAQ is way up. Enough said.
Texas

This is R3’s site in Georgetown, Texas. This is a skincare spa that was run by Megan DiMartino, who is an executive coach. This is what her own website says about this site:
“In 2005 I launched the award winning Novita Spa and Medical Rejuvenation Clinic, on the Georgetown TX. Square, in the Austin area. The Novita Spa and Medical Rejuvenation Clinic, combines Luxury Day Spa services with Clinical – Medical Facial and Body Contouring Treatments with Regenerative Stem Cell Therapy. All enhanced by the Novita Spa Clinical Skin Care Products. The Unique “Hybrid Spa Concept” was created!”
Arkansas

Not even going to comment here.
You Can’t Malke this Stuff Up!
So R3, after getting an FDA letter is still selling vials of “stem cells”. From looking at four of the clinics on their list, they are now selling these products to chiropractic clinics and day spas.
The upshot? How long can R3 keep this up? You would think they would be on an FDA shortlist for action, but so far it seems to be business as usual. As I often say, you can’t make this stuff up.
______________________________________________
References:
(1) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.
(2) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
