Can You Use the FDA Expanded Access Program to Treat Orthopedic Patients with Culture Expanded Umbilical Cord MSCs?
Over the past almost two decades, I have seen more creative attempts to get around the FDA’s biologic drug approval process than I can count. However, I had thought that with the FDA officially ending its regulatory discretion phase we had heard the last of umbilical cord companies trying to avoid a BLA. I was clearly wrong as a new angle just popped up on Linkedin called ‘Expanded Access”. So let’s explore the details of this program and if it can be used to deliver culture-expanded, umbilical cord MSCs to orthopedic patients.
What is a BLA?
A BLA is a Biologics License Application. This FDA approval pathway is also known as a 351. It’s an FDA approval that begins with an IND which is the application to start clinical trials. Just getting the IND is very rigorous. For example, we are preparing a product right now for our “pre-IND” meeting. From start to finish, this process will take us about 18 months and cost 6 figures as additional animal model data may need to be collected. Then there is a phase 1 safety trial, a phase 2 proof of concept, and a phase 3 multi-site clinical study. All of that can take 3-7 years and tens of millions of dollars with no guarantee that this investment pays off with FDA approval. In fact, most applications don’t get to that finish line.
Because it takes so long and is so expensive, many companies have reasonably asked the question, “Is there a way around this?” Through the years I have seen more creative angles than I can count. More recently in the umbilical cord tissue space, companies selling cord blood and Wharton’s Jelly tried to register their products as a 361 tissue rather than go through the 351 clinical trials pathway. The FDA shut that down, sending many Warning Letters (letters 1, 2, 3, 4 5, 6). It didn’t help that many of these companies were falsely claiming that their products contained live and functional “stem cells” (based on the published peer-reviewed literature) (4).
However, one of the constants of the “stem cell wild west” is that if you wait long enough, a new angle trying to get around a BLA will appear. This week on Linkedin we were all bombarded with the idea that you could use the FDA’s “Expanded Access” program to begin using culture-expanded umbilical cord cells, which are currently an unapproved drug product, to treat orthopedic patients before any final FDA approval had been granted. Is this legit? Let’s dig into the details.
An Umbilical Cord MSC and Exosome Manufacturer
An attorney who teaches entrepreneurship and reportedly owns the world’s largest umbilical cord stem cell culture expansion facility recently commented on mine and then Don Buford’s (who is an excellent orthopedic surgeon in Dallas using orthobiologics) Linkedin posts. The concept was that that there is an FDA pathway where we can use his product on orthopedic patients.
The attorney had contacted Dr. Buford about using his product, which was recorded by him and made publically available here:
Ultimately he posted this statement:
“I have been astonished by a series of LinkedIn chats with physicians who absolutely refuse to believe that there is a new pathway that can be used to treat patients with “serious or life threatening” conditions via Expanded Access for individuals or potentially groups of 100 to 1,000 patients
This was a legislative change targeting the cost of traditional Phase 1 – 3 trials for innovative cell therapy and other biotech firms willing to Sponsor individual trials under the FDA guidelines published in 2018 implementing the law passed in 2016.
This isn’t compasionate care or right to try – this is a distinctively different and unique pathway to an approval of Allogeneic stem cells – like the ones we produce at Regenerelle that are the Gold Standard of all cellular therapies. The approvals come in 24 hours up to 30 days maximum response time from FDA
It has been in the interest of many companies making products from autologous cells to claim that it is illegal to administer Allogeneic stem cells properly manufactured to patients under this program. They want to keep physicians scared of the FDA and scared of Allogeneic cells despite the fact they are safe and effective- more effective than autologous cells
It is hard for some people to admit they are wrong.”
So let’s break this statement down piece by piece. Before I do that, I apologize for the lack of brevity of this post, but this is a serious issue that deserves a deep dive. Why? Because if there is some FDA pathway that exists that would allow me to easily use culture-expanded cells here in the US with FDA’s blessing, I would be doing my patients a great disservice by not understanding how that works.
The FDA’s Expanded Access Program
So what happened in 2016? Legislation called the “21st Century Cures Act” was passed by congress (1). The goal was to create new pathways for FDA approval that would accelerate innovation. One of the most notable new programs that came out of that legislation was “RMAT” or Regenerative Medicine Advanced Therapy which is a slightly accelerated pathway for regen med products. We tried that route for culture-expanded cells around 2017 and got about 1/2 of the way through an IND application using a consultant who was convinced we could easily get this designation. Ultimately that consulting company fell apart and we were left having spent a bunch of money and not being convinced that an RMAT would be that much faster than the regular IND process. In the meantime, we licensed one of our culture-expanded technologies to a third-party company that is now beginning a phase two trial as part of a regular FDA IND. This is separate from the IND application that our company is now preparing for an allogeneic product.
However, another program that got approved in that legislation was the SPEA program, which is often called “Expanded Access”. SPEA stands for “Single Patient Expanded Access”. The idea behind the program is that with a simple application, in the right patient, FDA will approve the use of a drug that has not yet completed clinical trials. This is how the FDA describes this program (2):
“Sometimes called “compassionate use”, expanded access is a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.”
The application form that is filled out by the patient is called FDA Form 3926:

This looks pretty innocuous, but then so does the form for an IND, which takes about 12-18 months to get to a finished application with hundreds of pages of additional documentation.
There is an informative publication describing the University of Miami’s experience with the SPEA program (3). Note that they published on using this route for 10 patients with incurable diseases and from reviewing that paper, the good news is that the FDA will respond quickly to these requests. However, note that all of these patients had very serious problems ranging from spinal cord injury to brain injury to a rare nerve disease. In addition, IRB approval must be obtained for each patient/SPEA. That body will obviously review the risks versus benefits with that calculus being more favorable for approval if the disease being treated is severe and untreatable.
Could this Work to Treat Orthopedic Patients?
Given that the attorney on Linkedin was advertising his umbilical cord culture expanded MSC product to physicians who treat orthopedic and musculoskeletal diseases and had done so on a blog about knee arthritis, it’s reasonable to ask whether that diagnosis would qualify. Here I tested the requirements for being granted an SPEA against the diagnosis of knee osteoarthritis:
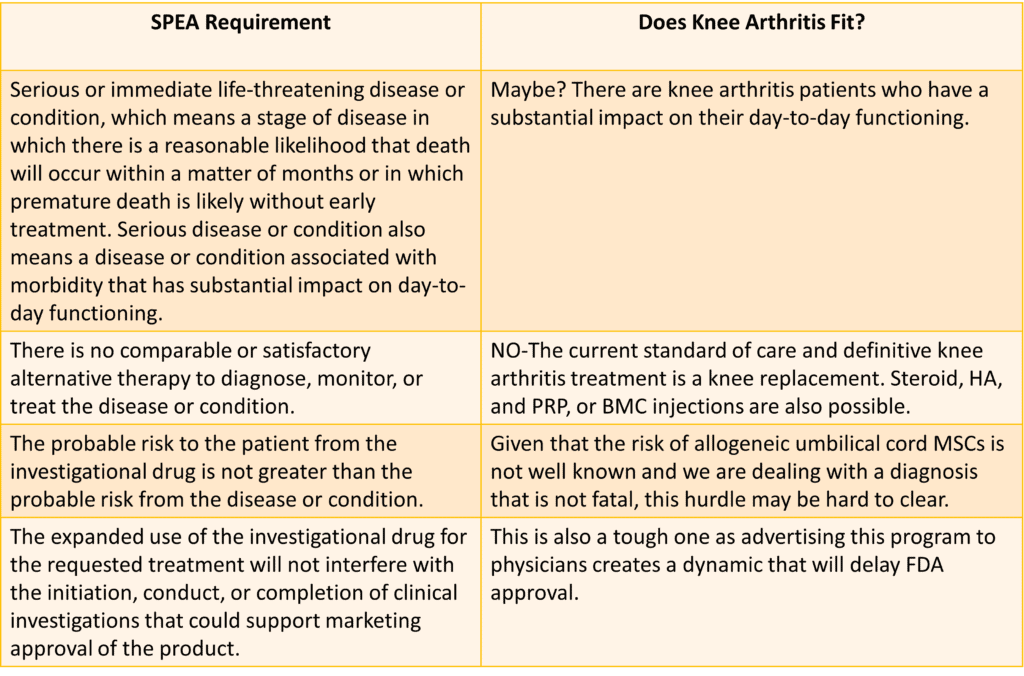
As you can see, there are lots of issues. The biggest is that there are many different existing treatments for knee arthritis. In addition, while we have a few clinical trials from outside the US on the use of culture-expanded MSC from umbilical cords, no experts I know would declare that the data supports that this treatment is “safe” as the FDA would define it. Finally, note that the FDA has included a very interesting clause that IMHO speaks to the problems of a manufacturer advertising this program to physicians.
FDA “Marketing Approval”
IMHO, one of the hardest things for physicians and entrepreneurs in this space to understand is that FDA is a “claims-made” system. The best example is charcoal. You can sell charcoal all day long to light your grill, but the moment you sell that same exact charcoal as a treatment for poisoning, it becomes an FDA-regulated product. Hence, the claims you make matter in the ultimate classification of your product.
Hence, making a claim that your product is “safe and effective” as a manufacturer, IMHO moves your product into that drug category. Physicians have a bit more leeway as the claims they make also overlap with state medical board rules. Both of these overlap with FTC rules.
Note that the FDA regulations for the SPEA I quoted above clearly state:
“The expanded use of the investigational drug for the requested treatment will not interfere with the initiation, conduct, or completion of clinical investigations that could support marketing approval of the product.”
So why is this clause here and what does it allow FDA to do? Realize that SPEA was designed because drug manufacturers didn’t want patients using their products that were still in clinical trials. Why? There could be a new adverse event that gets the product placed on hold or patients who would otherwise be candidates for clinical trials would be funneled through SPEA making completion of a clinical trial impossible. IMHO, what was never contemplated by FDA was that manufacturers would begin using SPEA to advertise that “‘individuals or potentially groups of 100 to 1,000 patients” could use this pathway”.
So how will FDA react when they find that a company is advertising SPEA directly to doctors and making the claim that this product type is “safe and effective”? IMHO they will use this language from their regulations and apply it in reverse. Clearly, advertising SPEA, in this case, could have the effect of bypassing an IND for this product. Second, IMHO if you really want to get the FDA angry, use the terms “safe and effective” for a product type that has yet to receive any FDA approval for any indication.
Another Big Hurdle
The original 21st Century Cures Act has this language regarding the Expanded Access program (5):
“APPLICATION.—This section shall apply to a manufacturer or distributor with respect to an investigational drug beginning on the later of— ‘‘(1) the date that is 60 calendar days after the date of enactment of the 21st Century Cures Act; or ‘‘(2) the first initiation of a phase 2 or phase 3 study (as such terms are defined in section 312.21(b) and (c) of title 21, Code of Federal Regulations (or any successor regulations)) with respect to such investigational drug.’’
Note the focus on the term “Investigational Drug” here. This is what the FDA page on the SPEA and FAQs for physicians states:
“Company (Industry)
Willing to provide the investigational medical product and either sponsors the expanded access, allows the FDA to cross-reference to their industry IND (for drugs and biologics) or IDE (medical devices) on behalf of the expanded access sponsor-investigator through the use of a letter of authorization, or provides the necessary investigational medical product information for the sponsor-investigator to submit to support an expanded access request.”
Note that to use this program the focus is on a product that has an existing IND application in place, meaning the FDA has already reviewed this product to some extent. There is an “or” above, which is also on the FDA 3296 Form:

Note that there are the elements here of having already filed an IND or having gotten pretty close (the rest concerns the ebb and flow of IND approval steps and holds). Hence, if you have just figured out your manufacturing process and have yet to file an IND, it looks like you’re being told to file an IND form. So will the FDA allow you to use the SPEA if an IND is not already on file for that product? I asked that question to an experienced FDA Regulatory attorney this morning and his take is that it would be very unlikely that the FDA would grant access to a drug that they have yet to approve through an IND or have yet to review for safety.
Here is the opinion of another regulatory expert (Scott Bruder, M.D., Ph.D.) from Linkedin this morning:
“There is a HUGE legal difference between a company that may have a facility that’s approved to provide stem cells, exosomes or other Section 351 biologic products (HCT/Ps) for use in the setting of a clinical investigation under an FDA-approved IND, and selling those products into the general community for clinical use. Do NOT fall prey to misleading company communications about Expanded Access or Compassionate Use in the setting of orthopaedics. There are NO allogeneic stem cell, exosome, or micronized birth tissue products currently approved for sale to treat ANY condition in the United States.”
Refuting Claims Takes a LONG Time
This blog was begun at 3:30 am and I finished around 7 am. Over the past two days, I also spent another 2 hours or so, between patients, researching this topic. Which has always been the problem. It takes only moments to make one of these claims and many hours to research whether that claim is accurate.
The upshot? Can you use the FDA SPEA process to treat orthopedic patients? Not based on my analysis of what the regulation actually says. Hence, my personal recommendation is for physicians who treat orthopedic and MSK problems to avoid using this minefield-laden pathway. At this point, having predicted everything from umbilical cord blood to Wharton’s Jelly FDA responses long before the FDA began sending Warning Letters, I think I’ve earned a bit of street cred in making sure my readers don’t step on FDA landmines.
______________________________________________________
Update: 7/12/23-Regenerelle did receive an FDA Letter; please see this link for that document.
______________________________________________________
References:
(1) USFDA. 21st Century Cures Act. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act Accessed 11/11/22
(2) USFDA. Expanded Access. https://www.fda.gov/news-events/public-health-focus/expanded-access Accessed 11/11/22
(3) Khan A, Bellio MA, Schulman IH, Levi AD, Longsomboon B, Brooks A, Valasaki K, DiFede DL, Pujol MV, Yavagal DR, Bates KE, Si MS, Kaushal S, Green BA, Anderson KD, Guest JD, Burks SS, Silvera R, Santamaria AJ, Lalwani A, Dietrich WD, Hare JM. The Interdisciplinary Stem Cell Institute’s Use of Food and Drug Administration-Expanded Access Guidelines to Provide Experimental Cell Therapy to Patients With Rare Serious Diseases. Front Cell Dev Biol. 2021 Jun 8;9:675738. doi: 10.3389/fcell.2021.675738. PMID: 34169074; PMCID: PMC8217825.
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.
(5) US Congress. 21st Century Cures Act. https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf Accessed 11/11/12.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
