A Study, a Professor, PRP and COIs Galore

Credit: Shutterstock
As I have written previously, you don’t have to look too deep for industry-academic connections in the recent spate of papers all using fake PRP and claiming non-efficacy. This morning we’ll look into the professor at the University of Sydney who published the recent knee OA paper in JAMA. Let’s dig in.
Why Are Conflicts of Interest in Medicine Important?
These past two decades, all sorts of rules have been put into place to let physicians and patients know about the conflicts of interest of researchers. For example, most universities have a strict conflict of interest policy. For a physician like myself, where’s it’s widely known that I’m the CMO of Regenexx, my conflicts are front and center. However, for university researchers, it’s tougher to tell if they have certain biases based on who’s paying the bills (or for their house). These biases are called Conflicts of Interest (COIs).
The Recent JAMA Knee Paper
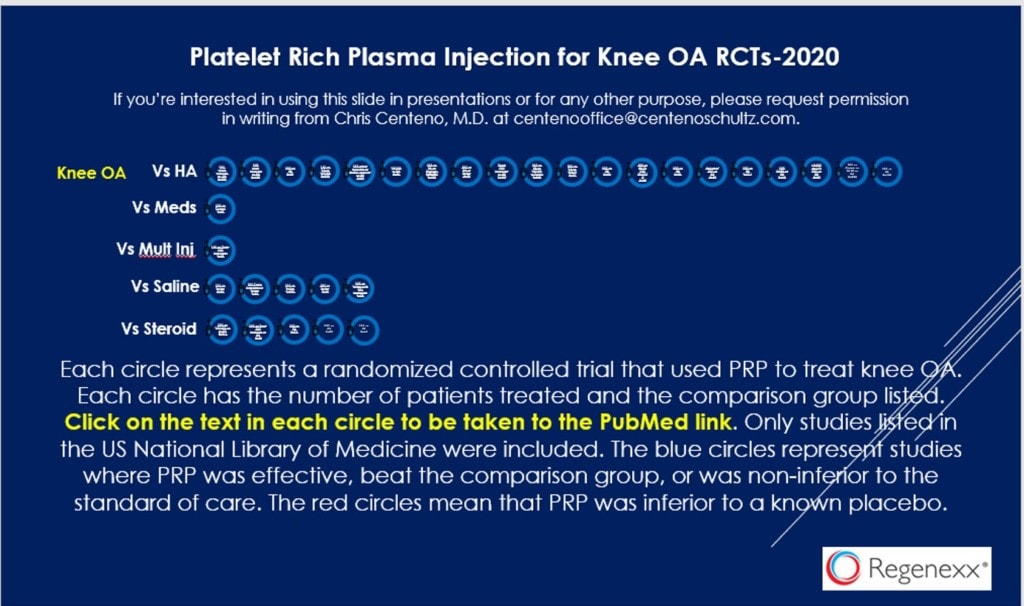
As of last year, there were 32 randomized controlled trials that show that PRP is effective in treating knee arthritis. Those are listed below:
However, last week a paper was published in the medical journal JAMA purporting to show that PRP injected to treat knee arthritis had no effect over placebo (1). The problem was that the authors used a PRP kit that couldn’t actually concentrate platelets. In fact, instead of the platelet count being several multiples of normal in the “PRP” they used it was within the range of whole blood. Now either the authors were totally oblivious to things like the PRP they used didn’t actually meet the minimum definition of PRP or there is some other explanation.
Other Explanations?
PRP is a real problem for scientists involved in the drug development pipeline for knee arthritis. If these new drugs are going to be financially successful, having dozens of RCTs showing that PRP already works and is far cheaper is a HUGE obstacle. It may well mean that their products will hit the market and never get insurance reimbursement.
I pointed out in a recent blog that the lead author of the JAMA paper wasn’t a physician who used PRP, but a Ph.D. not licensed to use this product. However, what I forgot was that in academic circles, the last author on a paper is the big fish running the show. That guy is also a Ph.D., but he’s not just any research scientist.
Who Is David Hunter?
I get emails from colleagues all the time telling me to look at this or that, so this email about the JAMA knee article was interesting:
“This rheum who planned prp to the Knee is a consultant for a liposomal dexamethasone product out of Taiwan.”
Who is “this rheum”? This is a Ph.D./rheumatologist from the University of Sydney. The interesting question posed by my reader is what is David involved in commercially?
Usually, the best place to look for a professor’s commercial conflicts is their university page, but I couldn’t find any conflict disclosures. So we need to dig deeper. So let’s look at the COI disclosure for Dr. Hunter from the JAMA knee paper:
“Dr Hunter reported receiving personal fees for scientific advisory board membership from Biobone, Novartis, Tissuegene, Pfizer, and Lilly. No other disclosures were reported.”
What are personal fees? Money, stock, and stock options that go from these five companies directly to David. Huh? That’s interesting. Why are these five drug companies paying David? To answer that’s let’s first perform a deeper dive to see if David has other COIs.
Oftentimes conflicts get listed on some papers and not others, so I cross-referenced the above list against a few of David’s other recent research article disclosures:
- October 2021 (2): “D.J.H. provides consulting advice for Pfizer, Lilly, TLC bio and Merck Serono.”
- November 2021 (3): “DJH reports personal fees from Pfizer, Lilly, TLCBio, Novartis, Tissuegene and Biobone outside the submitted work.”
- May 2021 (4): “DJH provides consulting advice on scientific advisory boards for Pfizer, Lilly, TLCBio, Novartis, Tissuegene, Biobone. CL has provided consulting advice for Merck Serono and Galapagos Pharmaceuticals, and receives research funding from numerous pharmaceutical companies (Fidia Farmaceutici, Inter-K Peptide Therapeutics Ltd, Taisho Pharmaceutical Co. Ltd, Concentric Analgesics Inc, Cynata Therapeutics, CEVA Animal Health, Regeneus) through specific services/testing contract research agreements between and managed by The University of Sydney or the NSLHD.”
Yikes! So far, based on this very limited review, Dr. Hunter and the university have taken money from at least 15 different pharma companies. Let’s explore just a handful of these to see if they have knee arthritis drugs in their pipelines:
- Novartis has recently received FDA fast track designation for a new drug called LNA043 to treat knee arthritis (5).
- Pfizer and Lily have been working on getting a new biologic drug approved for knee OA, but recently were turned down by the FDA (6). Dr. Hunter presented the results of their one-year study on this drug (tanezumab) at the ACR Annual Meeting in 2020 (7).
- TLCBio makes a liposomal steroid injectable to treat knee arthritis called TLC-599. David Hunter was quoted in a recent company press release as the principal investigator for this drug as stating, “Patients with osteoarthritis want a therapy that can provide fast onset and long duration pain relief so they can have a better quality of life. I am impressed with TLC599’s ability to consistently provide fast and durable pain relief in the majority of patients for the entire follow-up period of six months,” (8)
- Merck Serono has a knee arthritis investigational drug called sprifermin (9).
- Tissuegene has a drug candidate called Invossa which is a gene therapy to treat knee osteoarthritis (10). David Hunter is listed on the website as being on the advisory board for this company (11).
Super-Duper Conflicted
To say that Dr. Hunter has a few conflicts that might make him biased against PRP is a HUGE understatement. He’s super conflicted. Or hyper conflicted. Or MEGA conflicted. Meaning, I don’t know of another Ph.D. with as many sources of income and research funding from pharma companies. Or said another way, IMHO if these new drugs turn out to be no better than PRP, David’s personal financial fortunes will be drastically worse off.
Why Didn’t JAMA Catch This?
You would think the Journal of the American Medical Association would catch conflicts like this, as that should be their job, letting the public know when the research may have a bias. However, Dr. Hunter’s disclosures were buried in a difficult-to-locate section of the paper. In fact, only a handful of people who read that paper will ever look these conflicts up. One of the reasons may be that JAMA is one of the few journals that allows drug and device companies to advertise directly to physicians:
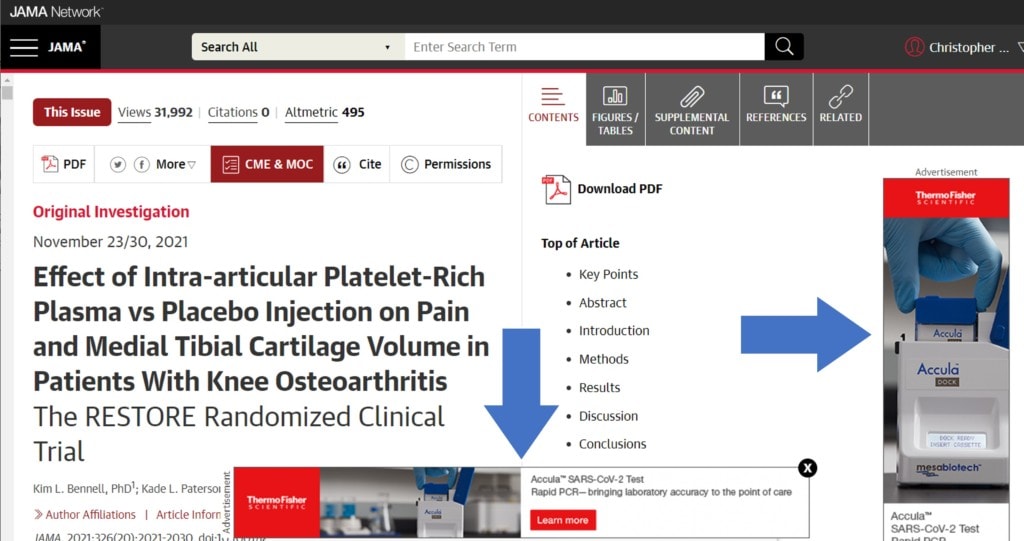
Do JAMAs advertising policies impact its editorial decisions? Or here’s a thought, how about a simple COI question like: “Will your personal finances (or those of family members) be made better, be unchanged, or improved by the findings you detail in this paper?”
The upshot? As I’ve said, the research scientists involved in drug approval pipelines for knee OA need to get rid of PRP. Hence, I suspect this won’t be the first or last paper authored by scientists who have a financial stake in the failure of PRP.
_________________________________________
References:
(1) Bennell KL, Paterson KL, Metcalf BR, et al. Effect of Intra-articular Platelet-Rich Plasma vs Placebo Injection on Pain and Medial Tibial Cartilage Volume in Patients With Knee Osteoarthritis: The RESTORE Randomized Clinical Trial. JAMA. 2021;326(20):2021–2030. doi:10.1001/jama.2021.19415
(2) Thomas A Perry, Xia Wang, Michael Nevitt, Christina Abdelshaheed, Nigel Arden, David J Hunter, Association between current medication use and progression of radiographic knee osteoarthritis: data from the osteoarthritis initiative, Rheumatology, Volume 60, Issue 10, October 2021, Pages 4624–4632, https://doi.org/10.1093/rheumatology/keab059
(3) Bandak E, Christensen R, Overgaard A, et alExercise and education versus saline injections for knee osteoarthritis: a randomised controlled equivalence trialAnnals of the Rheumatic Diseases Published Online First: 29 November 2021. doi: 10.1136/annrheumdis-2021-221129
(4) Oo WM, Little C, Duong V, Hunter DJ. The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date. Drug Des Devel Ther. 2021;15:2921-2945
https://doi.org/10.2147/DDDT.S295224
(5) Novartis. Novartis receives FDA fast track designation for LNA043 in osteoarthritis of the knee. https://www.novartis.com/news/novartis-receives-fda-fast-track-designation-lna043-osteoarthritis-knee Accessed 11/30/21
(6) Fierce Biotech. FDA panel knocks down Pfizer, Lilly’s osteoarthritis pain drug in near-unanimous vote. https://www.fiercebiotech.com/biotech/fda-panel-knocks-down-pfizer-lilly-s-osteoarthritis-pain-drug-near-unanimous-vote Accessed 11/30/21
(7) American College of Rheumatology. Meeting Abstracts. ABSTRACT NUMBER: 1641-Clinically Important Improvements in Patients with Osteoarthritis Treated with Subcutaneous Tanezumab: Results from a 56-Week Randomized NSAID-Controlled Study. https://acrabstracts.org/abstract/clinically-important-improvements-in-patients-with-osteoarthritis-treated-with-subcutaneous-tanezumab-results-from-a-56-week-randomized-nsaid-controlled-study/ Accessed 11/30/21
(8) TLCBio. pressroom. Press Releases. Taiwan Liposome Company Reports Positive Top-Line Data from Phase II Clinical Trial of TLC599 in Knee Osteoarthritis Pain. https://www.tlcbio.com/en-global/press-releases/detail/News_20180820 Accessed 11/30/21
(9) Merck KGaA. Press Releases. Merck KGaA, Darmstadt, Germany, Announces JAMA Publication of Phase II Results of Sprifermin for Osteoarthritis Structure Modification. https://www.emdgroup.com/en/news/jama-08-10-2019.html Accessed 11/30/21
(10) Kolon Tissuegene. INVOSSA. https://www.tissuegene.com/en_US/product-pipeline/applications Accessed 11/30/21
(11) Kolon Tissuegene. Scientific Advisory Board. https://www.tissuegene.com/en_US/about/advisory-boards Accessed 11/30/21

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.