Fibrin Treatment for Back Pain: Do You Need to Seal a Degenerated Disc?
This week, while I was performing a procedure on another patient, Dr. Bashir of Regenexx Miami poked his head in to let me know of his great success with a chronic disc pain patient. It was an impressive 95% improvement in a patient who had tried it all and was now back to golf after a stem cell injection into the disc. As he told me about this, I thought that this would be a great way to illustrate why fibrin treatment for back pain to seal the disc in most patients with chronic back pain makes little sense.
Understanding the Disc
Axel_Kock/Shutterstock
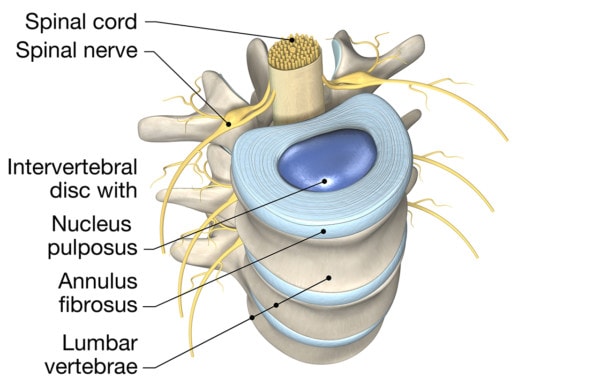
The first thing you have to understand about the disc is how it’s made and what it does. It’s basically a shock absorber between the spine bones that also allows motion. It has a soft inner gel (nucleus pulposus) surrounded by a tough outer fibrous covering (annulus fibrosis). As we age, the cells inside the disc lose the ability to hold onto water and begin producing less of a chemical that facilitates that function. This means that older discs can become dried out and collapse, becoming rather inhospitable places for cells to live. Add to this that the stability of the spinal segment around the disc begins to get worse and worse due to muscle atrophy and loose ligaments, and the disc can get beat up. Tears can develop in the outer covering.
The Concept of Needing to Seal the Disc
Some physicians, based on the teaching of a Texas doctor, are now beginning to offer disc-sealant injections with fibrin. The type of fibrin they’re using is sort of like natural rubber cement. You can inject it into the disc or its tough outer covering and it will “seal” the small tears and holes in the disc, preventing stuff from leaking out. The reason given for doing this is that sometimes discs can leak chemicals, which can cause inflammation and swelling around the spinal nerve that passes by the disc (a.k.a. chemical radiculitis), leading to back or leg symptoms. Hence, advocates for this procedure contend that sealing the disc prevents that from happening. In addition, they argue, if you’re going to be injecting stem cells inside a disc, sealing it first will keep those cells in that structure.
The before and after images of injecting really anything that gels up in the disc are impressive, whether that be fibrin, a same-day bone marrow stem cell procedure, or platelet rich plasma. Basically, the disc plumps up. This is because all of these things contain fibrinogen, which then causes fibrin to be laid down. However, there’s little evidence that a severely degenerated disc that has lost its height stays at the the new taller configuration. Basically, once the new fibrin is dismantled by the body (which takes a 1–2 weeks), the disc returns to its collapsed configuration. So the first thing to be cautious about is the X-rays showing the restoration of normal disc height immediately after after a fibrin or other injection, as the height doesn’t last!
Fibrin Treatment for Back Pain: More Misleading Research About Sealing the Disc
My prior blog on the Pauza Discseel™ procedure found many inconsistencies between what the website suggested and reality. The biggest was that while the website would make you believe a randomized controlled trial (RCT) had been performed that supported that the procedure was effective, in fact the only RCT performed showed that the procedure was no better than a placebo shot. In addition, the website reasoned that you needed to seal the disc to keep injected stem cells inside because stem cells can cause cancer. No research shows this, and the website erroneously points to a “respected Johns Hopkins study” without any such study actually existing. In fact our recent 2,372-patient stem-cell-safety study included patients with stem cells injected into their discs, and no such issues were found.
The website about using fibrin treatment for back pain to seal discs has so much misleading information that it’s almost difficult to know where to begin. However, this morning I’d like to highlight another study brought to light on the LinkedIn discussion board where we directed our readers to participate in the discussion about the technique.
There is a claim on the website that tries to scare patients off from using stem cells to treat their discs that states that stem cells injected into the disc can cause bone spurs to form (if they leak out). The site states that this is therefore one pressing rationale to seal the disc before injecting stem cells. This claim has issues on many levels, not the least of which is that the disc will only stay sealed for about a week to 10 days. However, like many things said on the fibrin disc-sealant site, there’s what the words superficially suggest and then reality once you take a peek under the covers.
In this case of bone spurs, the website refers to a rabbit study where the authors tried hard to get the stem cells to make bone. Cells were cultured in a special media to force them to make bone and were not the treated rabbit’s own cells, and the injury to the disc was not one that in any way replicates the real human degenerative disc disease condition. In fact, the stab model used to injure the disc before the stem cells were injected is about the same as taking a ball-point-pen-sized implement and jabbing it into your disc and sucking out all of the living cells. So unless you’ve been recently abducted by aliens and had your disc sucked out, the starting point for this experiment was nothing like your degenerated disc! In conclusion, nothing about this study is in any way like the same-day stem cell based disc injections that patients are receiving for torn and painful discs.
Our Patient
To illustrate why sealing the disc with fibrin is not needed, a great example this morning is Dr. Bashir’s patient. Ted had a multi-year history of low back back pain and had failed physical therapy and epidural steroid injections. He was so disabled that he couldn’t throw a Frisbee with his kids. His MRI had a tear in the back of the disc, and his discogram (a test where dye is injected into the disc to see if it’s painful) showed that the L5–S1 disc was painful. In addition, that test showed L5–S1 was a leaky disc in that the dye squirted right out. The L4–L5 disc was also leaking, but it wasn’t painful.
Dr. Bashir injected the patient’s own stem cells using our proprietary Regenexx same day stem cell procedure in May of 2015, and he reported 75–80% improvement. Since he wanted more, the disc was injected a second time in November of 2015. In both instances, the L5–S1 disc was injected with his own stem cells (and NOT sealed with fibrin). The patient did well and was just seen this week for his one-year follow-up. He’s now reporting a 95% improvement! He’s back to all activities and without pain, and never once did we entertain the idea that we needed to first seal his leaky low back disc.
Why did this work so well if the disc was leaking? As discussed above, a same day bone marrow stem cell procedure has its own natural fibrinogen from the patient’s own body. It seals the disc all by itself. Unlike fibrin glue, which has been shown to be a very poor stem cell scaffold because it’s too dense, bone marrow sets up its own natural scaffold for stem cells immediately upon detecting exposed collagen fibers, like those seen inside a torn disc.
The Very Real Downside of Injectable Fibrin
A few years back, we saw a woman who was a chronic dural leaker. This meant that the covering of her spinal cord (the dura) that was supposed to hold back cerebrospinal fluid (the liquid in which the brain, spinal cord, and nerve roots are suspended) would spontaneously spring leaks. She had been treated with the same type of fibrin that doctors are now using to seal the disc. There was just one little problem. Despite the product being FDA approved for being safe and free of communicable disease, she had been injected with a bad batch made from a blood donation of a patient with hepatitis C. After that therapy, she had developed the disease. While we were able to help her using highly concentrated platelet rich plasma injections instead of fibrin, she serves as a very real reminder that there’s nothing like the power of your own tissues, as even FDA-approved biologic tissues have a false negative rate when screening disease. In addition, while this is a rare event, it’s not rare if it happens to you.
The upshot? So is there a pressing need for fibrin treatment for back pain to seal the disc before injecting stem cells? Not really. Certainly nothing on the fibrin disc-sealant site sounding alarm bells of why sealing the disc can prevent catastrophe is remotely credible. In addition, cases like Ted’s show us that despite having a severely leaky and painful disc, without fibrin being injected, the right patients with a torn and painful disc do great. Finally, our dural leaker patient is a stark reminder that using your own tissue to heal your body should always be your first choice!

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
