Is There a Stem Cell Therapy Age Limit?
A question that I get asked all the time is whether there is a stem cell therapy age limit. The reason why I get asked this question is because of a massive misinformation campaign put out by clinics illegally selling fake stem cell therapies. Let me explain.
Our History with Stem Cell Treatments
We began using stem cells to treat orthopedic conditions in 2005. Since we’ve tracked those patients in a registry, we have been able to look at the effects of age on thousands of patients who used their own bone marrow stem cells to treat everything from knee arthritis to shoulder rotator cuff tears. Outside of a single diagnosis (hip arthritis), none of those treatment outcomes got worse with age. We have lectured on this widely and published many peer-reviewed articles on this data.
A New Sales Narrative
About 5 years ago we began to see non-physician clinics enter this space. They advertised that they used “stem cells” from amniotic or umbilical cord sources. They would also claim that these products had millions of young and healthy stem cells and that patients who were older had too few good stem cells to effectively heal or manage orthopedic problems. Meaning that they would tell patients that there was a stem cell therapy age limit.
Testing “Stem Cell” Products
Before these clinics began using these birth tissue-derived products, we were approached by a sales rep in 2014 about testing an amniotic fluid product that he claimed had millions of live stem cells. The same sales pitch was given by the sales rep about a stem cell therapy age limit. We tested this product in our lab and found no living cells at all, let alone stem cells. Since then our lab and several universities have tested multiple amniotic and umbilical cord products and found that they all have dead cells with no living stem cells (2-4).
Testing Bone Marrow Stem Cells from Older Patients
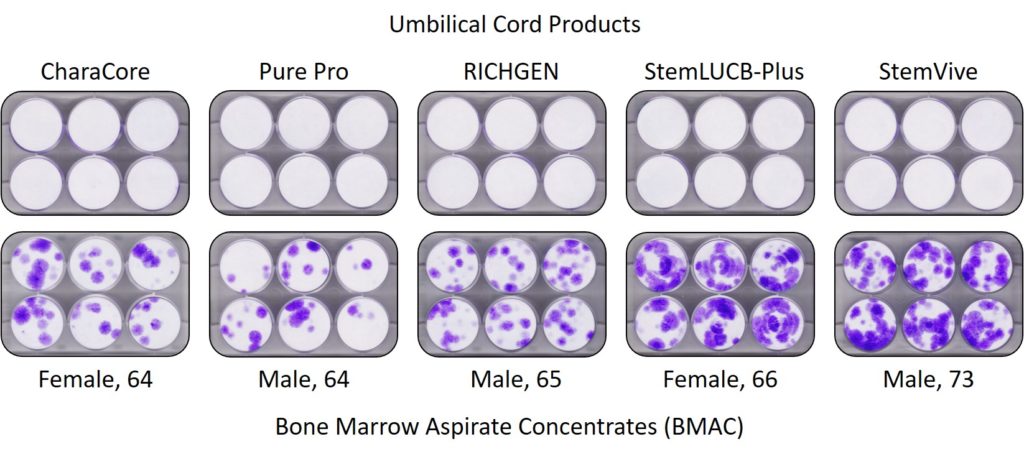
The picture below is worth a thousand words:
This is a lab test of the stem cell therapy age limit by looking at the stem cell content of umbilical cord products commonly used by clinics. All of these products claimed to have high numbers of live stem cells compared to the few that could be found in middle-aged and elderly bone marrow. The top row with the all pristine white means that none of these umbilical cord products had any stem cells. The bottom row with the purple dots clearly shows that middle-aged and elderly bone marrow has live and active stem cells.
Why is This Happening?
If tissues like umbilical cords do have live stem cells, why are these products showing up with dead stem cells? Because if you take an umbilical cord from a live birth and rush it to the lab and place those cells into culture, they’ll survive. However, if you take that cord, package and ship it to a processing center, let it sit, then process it, then bottle those cells, freeze them and then store them, eventually ship them, and shock thaw them in the doctor’s hand-nothing much survives.
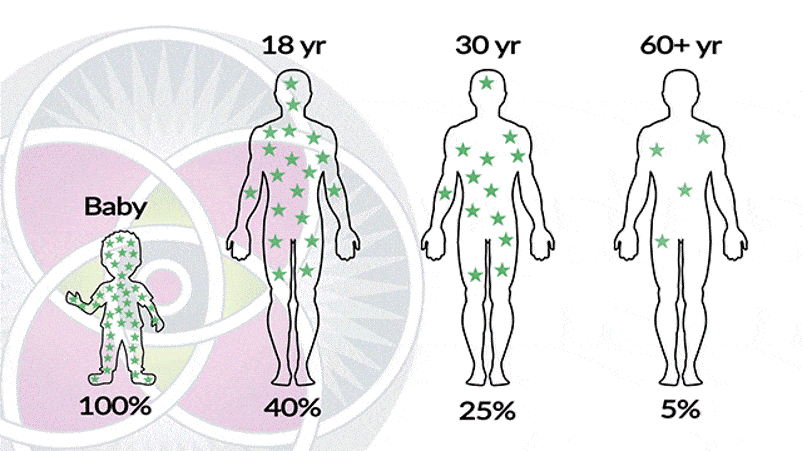
A Crazy Cool, but Misleading Graphic
I love graphics that are simple and convey a lot of medical information. I found this one on the website of a clinic that had just received an FDA letter for illegally claiming to use umbilical cord stem cell products. The above diagram purports to show the number of stem cells in a baby versus an 18-year-old and then all the way to someone who is 60+ years old.
First, you now know that all of the “stem cells” in the products used by this clinic and others like it are dead. Hence, any assertion that the clinic is giving patients lots of living and active stem cells like that baby picture off on the left is false.
In looking a second time at the graphic, you don’t need a Ph.D. to see there’s a problem. For example, 18-year-olds and 30-year-olds have no issues healing most any injury. So if you believed the graphic accurately shows stem cell density in the body at various ages (it doesn’t-more on that below), you wouldn’t be able to explain how a good innate healing ability at age 30 can occur with only 25% of the original stem cells.
To get a bit more scientific, the data they’re attempting to show you is based on a few small studies that include newborn babies that looked at stem cell density in the body. However, when our lab tried to repeat that analysis with just adults and a much larger number of patients, there was no correlation:
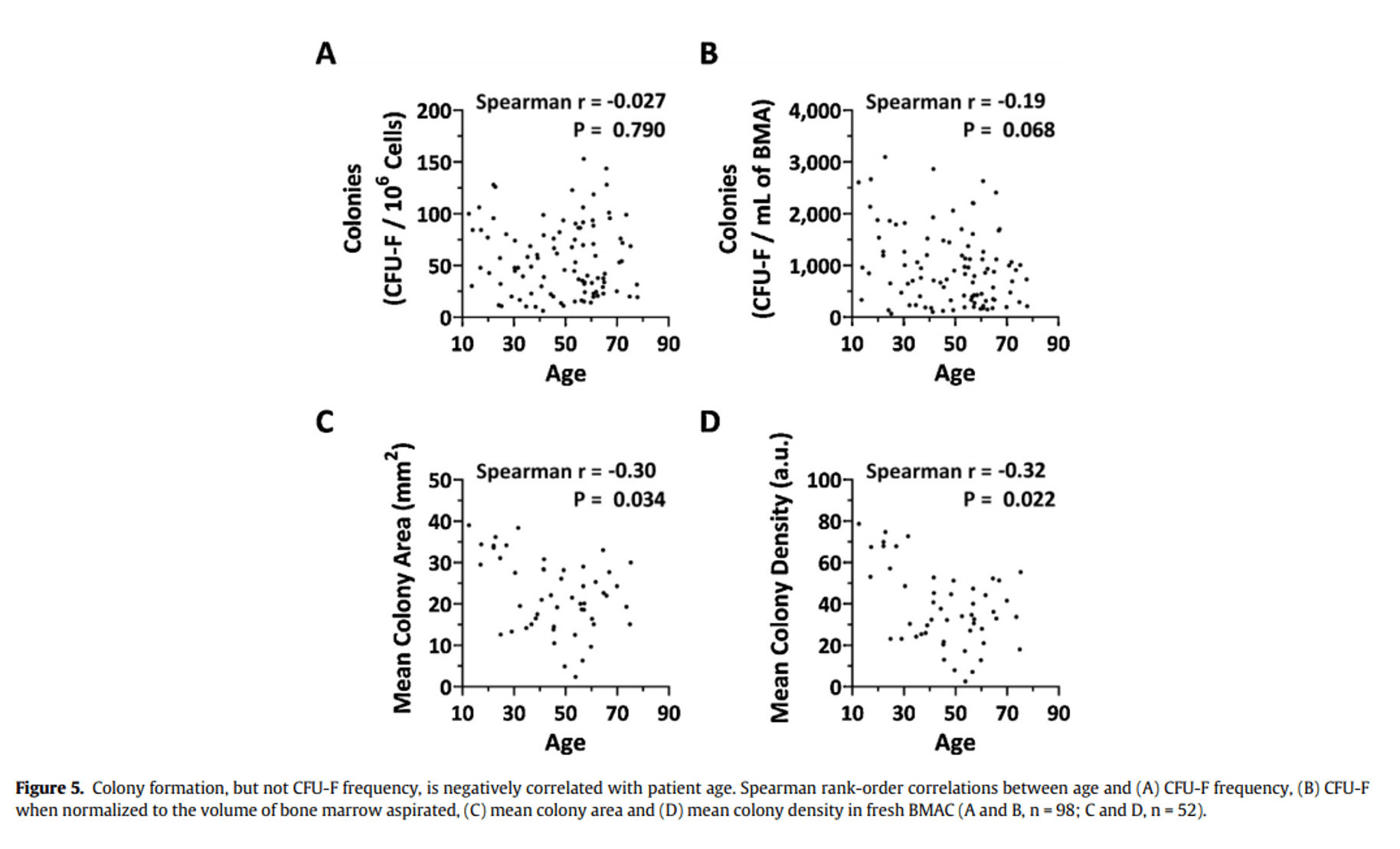
This is from our recent peer-reviewed publication. You’re looking at plots of the CFU-F (stem cell number) in bone marrow, versus age (1). If there was a nice tidy correlation like the diagram shows, we should be seeing a plot that looks like this:
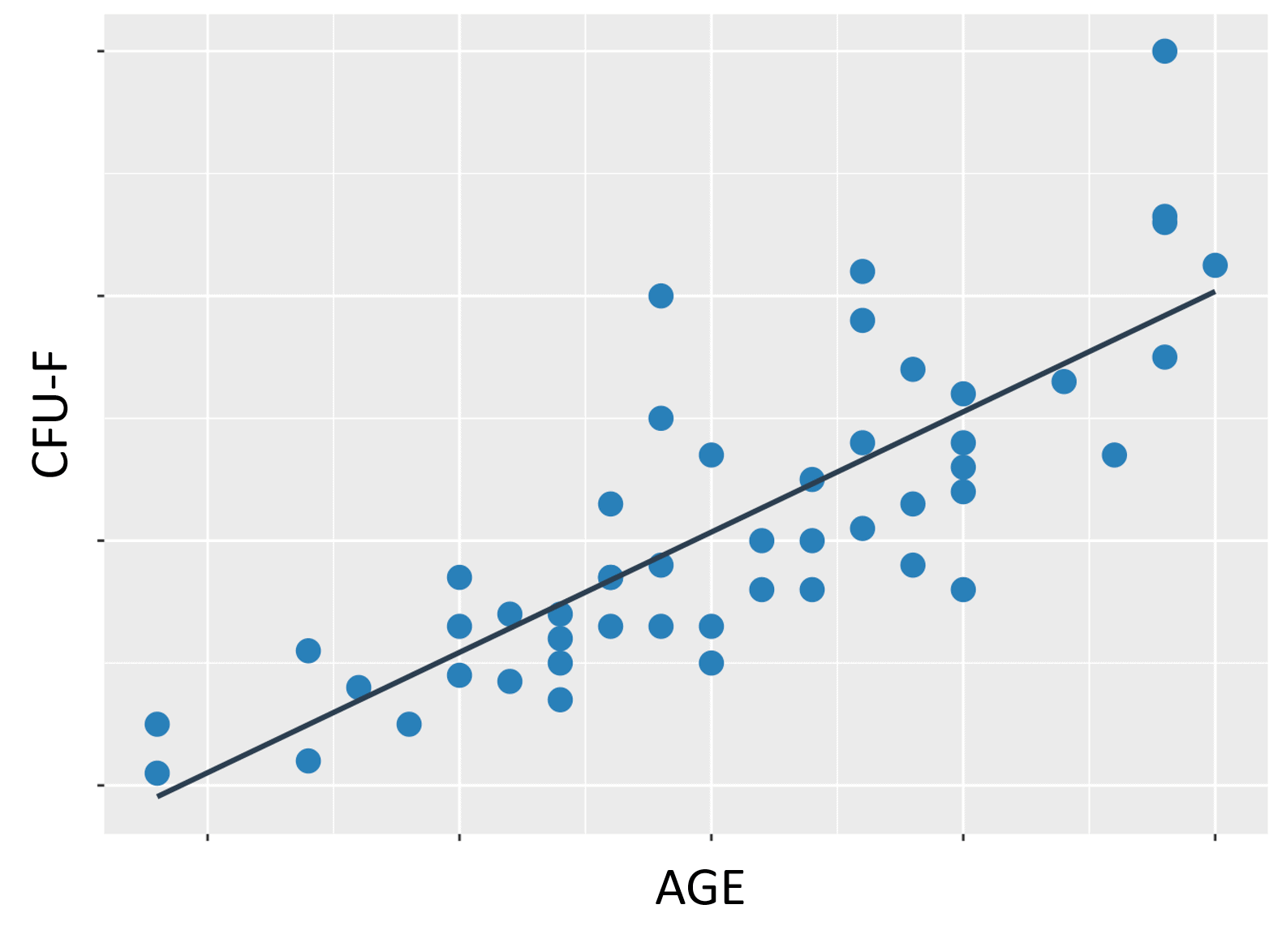
However, the points in our plots don’t follow this nice, tidy line and are all over the place. Hence, there is a poor correlation between stem cell number and age.
How Does Age Actually Impact the Stem Cells in Bone Marrow?
Once we looked at our data a few different ways, the correlation that does exist is stem cell activity. Meaning older cells tend to grow less quickly in culture than younger cells. Hence, if you’re older and using your own stem cells, how is that addressed? Very simply through concentration and precise placement.
First, as I always tell patients, if your stem cells didn’t work you’d be dead. Meaning, stem cells in our body constantly repair all sorts of daily damage. If I suddenly got rid of all of them through some magical experiment, you would only last about 1-2 months before you succumbed from that day to day damage.
Second, if you don’t have enough stem cells in one body area to facilitate repair, then all we need to do is to get them from a stem cell-rich area (like the bone marrow), concentrate them, and then precisely deliver them back to where they’re needed. This is why things like the quality of the bone marrow aspirate, the degree of concentration of the sample, and the precision with which it’s delivered back to the right spot (using imaging guidance) are such a big deal in stacking the deck in your favor.
A Practical Example
Yesterday I treated a patient that shows that there really is no stem cell therapy age limit. He was 88 when he was first treated for severe knee arthritis. He got great relief for about a year and a half and was back for a booster treatment at 90. His cell counts really weren’t any different than the younger patients we treat. However, because of that cell activity difference, I performed a high cell number bone marrow aspiration and we concentrated his cells to highest number possible. I then precisely injected those into the right areas using x-ray and ultrasound guidance.
The upshot? There really is no stem cell therapy age limit. While the above graphic is cool, it’s also misleading and not supported by the existing published best literature. In addition, the idea that clinics are delivering lots of live and active young stem cells from birth tissues like amniotic fluid/membrane or umbilical cords is false. These are dead tissues. Hence, if you want a live stem cell treatment, that’s limited to using your own bone marrow, concentrating what’s there, and delivering that precisely back to the spot where it’s needed.
____________________________________
(1) Berger DR, Aune ET, Centeno CJ, Steinmetz NJ. Cryopreserved bone marrow aspirate concentrate as a cell source for the colony-forming unit fibroblast assay [published online ahead of print, 2020 Jun 18]. Cytotherapy. 2020;S1465-3249(20)30619-8. doi:10.1016/j.jcyt.2020.04.091
(2) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.