Lipogems Review: Is This a Fat Stem Cell Procedure?
Lipogems is a fat-processing kit that has gained some popularity the past few years. Near as I can tell, it produces a beautifully cleaned fat graft that is finely chopped. However, many physicians have begun to call this a stem cell procedure. Is that true?
What Is Lipogems?
Lipogems is a device used to process a fat graft. The doctor first performs a liposuction and then places the fat inside a chamber that has many small steel balls. There are a few steps, but suffice it to say that the doctor shakes the fat in the device and the steel balls macerate the fat while saline cleans it. It’s a pretty cool system that’s been likened to a cocktail shaker.
What the doctor gets out of the system is tiny pieces of fat that can be drawn up into a syringe and injected. This is then supposed to be used surgically for structural support (e.g., filling wrinkles or buttressing skin), but the focus of the device’s marketing at the conferences I attend has been in treating musculoskeletal injuries (e.g., like knee arthritis) through injection. Before I get into our lab review of this product and whether it’s a stem cell procedure, let’s review its regulatory status.
Lipogems Regulatory Review
I’ve been told by many physicians that the Lipogems kit has an indication for treating knee arthritis. Is that true? The company was given a 510K device approval. For those that don’t know, a 510K is a “substantial equivalence” determination. Meaning that there is some product on the market that existed before medical devices were regulated by FDA in the 1970s and Lipogems made a claim that its product was just like that product. This is what the FDA approval says:
“The Lipogems System is a sterile medical device intended for the closed-loop processing of lipoaspirate tissue in medical procedures involving the harvesting, concentrating and transferring of autologous adipose tissue harvested with a legally marketed lipoplasty system. The device is intended for use in the following surgical specialties when the transfer of harvested adipose tissue is desired: orthopedic surgery, arthroscopic surgery, neurosurgery, gastrointestinal and affiliated organ surgery, urological surgery, general surgery, gynecological surgery, thoracic surgery, laparoscopic surgery, and plastic and reconstructive surgery when aesthetic body contouring is desired. ”
Hmmmm…Nothing about knee arthritis or any type of arthritis or tendon injury. Nothing about injections. Nothing about stem cells. Reading between the lines, the 510K device approval that the company was granted was to concentrate a fat graft for use in all types of surgery.
So How Did We Get from a Surgical Fat Graft to Stem Cell Injections to Treat Arthritis?
First, let me say that I have seen no evidence that the company that makes Lipogems is calling this a stem cell procedure. While the company is positioning itself in the knee osteoarthritis market, the “stem cell” moniker is largely being added by physicians. Why?
There is a stem cell procedure called stromal vascular fraction, or SVF, that has been placed into the cell-drug category (i.e., not allowed to be used by physicians at this time). In this procedure, the stem cells and many other cell types are separated from their collagen prisons inside adipose tissue and further cleaned and concentrated. Given that the FDA has been cracking down on the physicians using this fat stem cell procedure, this left a gaping hole in the fat stem cell world. We began to see physicians as far back as 2013 calling some micronized (i.e., finely chopped) fat grafts stem cell procedures. I’ve blogged on our inability to find many stem cells in these fat-graft procedures. Hence, Lipogems came to market 2–3 years ago, right when it began to become obvious that SVF was being shown the door, so it became the new defacto fat stem cell procedure.
The Lipogems stem cell train also really got rolling in a number of lectures given at national conferences. At those events, research was presented that seemed to show that the Lipogems system chopped the fat so finely that the stem cells could literally crawl out of the adipose tissue in culture. Hence, the seed was planted in the minds of physicians.
Finally, I’ve seen a local orthopedic surgeon here advertising Lipogems as a stem cell procedure. It’s also not hard to find physicians advertising it this way all over social media. In addition, the number-four suggested search result under Google is “Lipogems stem cell”; hence, we likely have patients searching for it this way. See the video for more details.
Now let’s explore whether this is true, kinda true, or totally false.
Our Lab Testing for Stem Cells
First, we requested samples from the company that manufacturers these kits. While the rep was fine with our testing the device, the company blocked us from getting a kit. Hence, it was clear that they didn’t want us testing this product. Hence, we had to purchase these through a third-party physician who has used the system, and we had to pay full price (they weren’t cheap).
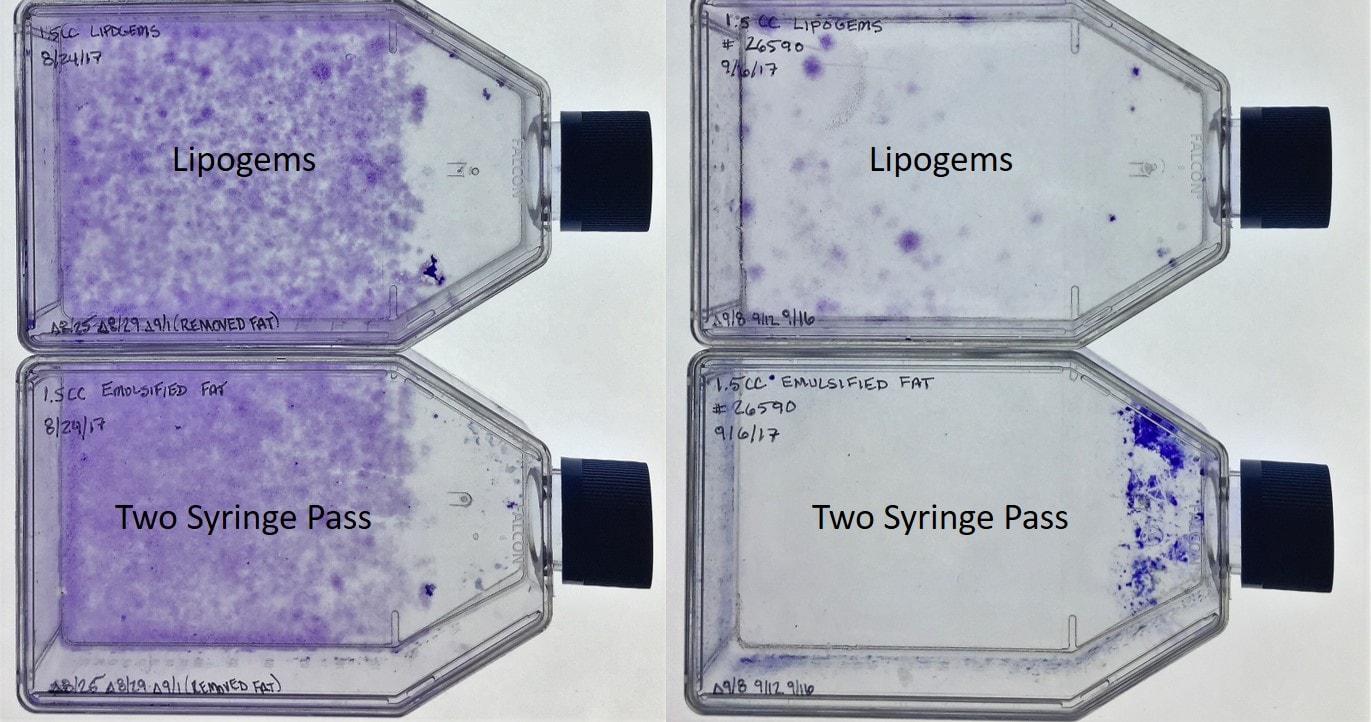
We processed two patient samples with the Lipogems 60 ml kit. We did not have a sales rep there for obvious reasons, but we did have a physician who had used the kit before. We compared the output of the kits to a simple and common fat micronization setup. Basically, this was two syringes hooked up to an inexpensive mechanical emulsifier system. In this system, you just pass the fat back and forth between two syringes with the device in the middle. The device has various size grates with the goal being to reduce the size of the fat particles further with successively smaller grates.
As you can see above, the results are pretty easy to ascertain. The dots in the flasks represent stem cell colonies for patient 1 on the left and patient 2 on the right. The more dots, the more stem cells. This is the CFU technique I’ve blogged on in the past. Basically, there is no difference in the small number of stem cells liberated by both techniques. There is, however, a huge difference in the price of the two procedures. The disposable Lipogems kit typically runs more than a thousand dollars and sometimes up to two thousand. The mechanical emulsifier system on the right is autoclavable and in treating 100 patients would cost only several dollars per use. In fact, we have now also compared these CFU counts to a simple $1–2 disposable syringe to syringe luer lock connector and found no differences.
So Is Lipogems a Stem Cell Procedure?
To make that determination, you also need one more piece of information. That is that the stem cell numbers obtained from 60 ml of fat processed with the two Lipogems kits and the mechanical emulsification system (Two Syringe Pass) were only a tiny fraction of the number of stem cells obtained from bone marrow concentrate (BMC). So is this a stem cell procedure? While some stem cells likely get knocked loose in the processing, our data is showing that most of the stem cell separation from collagen likely happens from the liposuction procedure itself. Hence, given the low number of cells and the fact that we don’t know if this low stem cell content is tied to outcome, I would say that Lipogems is not a stem cell procedure. This is different from BMC, where we have several studies tieing stem cell content to clinical outcome.
Centeno the Buzzkill!
I remember my teenage nephew and niece used to have their mom’s phone number in their phones as “Buzzkill.” Hence, every time Mom called, “Buzzkill” would show up on their caller ID! This is a bit what I feel like as overhyped technology after technology is tested by our research lab and then doesn’t live up to the hype that physicians have assigned to it. Why? There’s only so much that can be done within the FDA’s minimal manipulation, or 361 tissue, guidelines. Hence, when salespeople or physicians ascribe magical properties to the latest and greatest regen med trend, it’s not hard to show that it isn’t magic and never was magical.
As a disclaimer, I will say that I have several colleagues who use the Lipogems system and think it can be used to help patients with knee arthritis and tendon injuries. One of these providers has been diligently collecting data, and I applaud that effort. What I’ve published in this blog doesn’t change that in any way. The focus of this piece was whether we should be calling Liogems a stem cell procedure.
The upshot? Based on the data we have, Lipogems shouldn’t be advertised as a stem cell procedure. I know the company that manufacturers the unit agrees with me on that concept. However, advertising the output of this kit as “stem cell therapy” is rampant. So will the company start to self-regulate and refuse to sell kits to physicians who are crossing this line? Time will tell.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.