SI Bone? Is This a Good Thing to Do to a Patient?
There’s a treatment for SI joint instability made by a company called SI bone which makes a minimally invasive implant that is, in my opinion, a maximally invasive way to treat SI joint instability. Recently I stumbled across public FDA documents on the disturbing complications reported for this procedure that was eye-opening. Let’s dig in.
What is SI Bone?
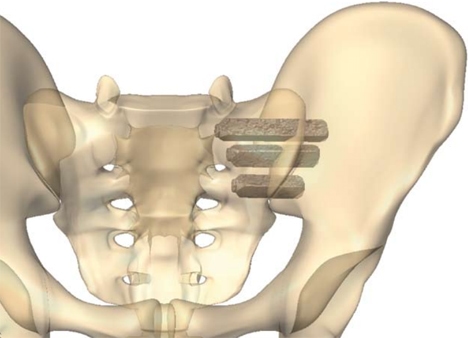
SI Bone is the name of a company that makes an SI joint implant called “IFuse”. The goal of the procedure is to take a patient with a painful SI joint and fuse that joint solid using usually three large titanium rods that are placed through the joint (image here). The company got FDA device approval in about 2016 and then published clinical studies and claims that the procedure has been used 40,000+ times as of 2019.
What is SI Joint Instability?
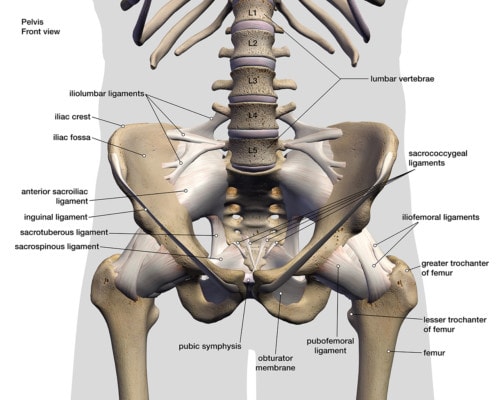
The SI Bone iFuse system is designed to fuse an unstable SI joint. What’s that? the SI joint lives between the tailbone (sacrum) and the side of the hip (ilium). The joint is surrounded by thick ligaments and these can become damaged due to trauma like a fall or a car crash or loose with pregnancy. If they’re loose, the SI Joint can move around too much, leading to pain.
Hank Grebe/Shutterstock
How Successful is SI Joint Surgery?
As a physician who has successfully treated thousands of patients with various levels of SI joint instability, this is where I believe reality and hype are swirled together to get information that likely isn’t very accurate. The company, of course, has published several studies with reportedly high success rates. Having said that, prior attempts at SI joint fusion in the late 90s were a dismal failure. So how can these two pieces of information be harmonized?
Let’s dig in. The early research published on the iFuse implant was 1-2 year follow-ups of patients that had been previously enrolled in a randomized trial (RCT) which had a 6-month follow-up [1,2,3]. The longer two-year research study that wasn’t part of the RCT seemed to report positive results. However, how good the results really were is arguable. For example, before the iFuse implant was banged through their SI joints, about 3/4s of the SI joint pain patients needed to take narcotics and 2 years after this invasive procedure, a bit more than half were still taking narcotics! Given the very nasty side effects caused by taking opioids over the long run, reducing use by just 21% with more than half of the patients still addicted is not that spectacular.
What are the Risks of the SI Bone iFuse Implant System? The 30,000 Foot View
Regardless of what the success rate really is or isn’t, the big issue that I have with this system is the risk to the patient. From a 30,000 foot viewpoint, they’re taking three titanium implants the size of a pen and banging those through the SI joint. What could go wrong? Turns out quite a bit.
The first issue is obvious. I love simple illustrations of risk. Below you see the 7mm SI bone iFuse implant next to a 22 gauge and 25 gauge needle with both shown approximately to scale. The iFuse Implant is placed surgically after a drill and reamer destroy the SI joint by creating a hole in it. The implant you see below (about the size of a ballpoint pen) is then placed into that hole. This happens 2-3 times each side.
Compare that to the needles you see. These are being shown because less invasive treatment for SI joint instability involves injecting prolotherapy solution, platelet-rich plasma, or bone marrow concentrate through the needles you see below into the ligaments that surround the SI joint. These needles are guided by x-ray or ultrasound or both. Note that both are tiny compared to the iFuse implant device.
You see, the amount of damage you do with your procedure is directly related to the complication rate. Meaning, more damage means that more severe things can go wrong. So let’s dig in further on that topic.
The Research on SI Bone iFuse Complications
The cited studies that were paid for by the company state that only about 5% of the patients had significant complications, but is that really accurate? A different study that was not paid for by the company disputes that complication rate [5]. This database was derived from insurance claims on complications from more than 400 SI joint fusion procedures. The complication rate was three times higher than the data reported in the company-sponsored study (4.7% vs. 16.4%) [5]! A common side effect noted in this insurance research was “new spinal problems”. Let’s jump into that.
In another company-sponsored research study, the SI-Bone fusion device was used in about a hundred patients (4). When reading the fine print, I noticed this statement:
“In total, 114 adverse events were reported between years 3 and 4; however, none were rated as probably or definitely related to the study devices or index surgical procedure.” Why did this statement capture my attention? Because this next part worries me: “Many events indicated underlying degenerative disease associated with age and osteoarthritis (eg, hip, knee, shoulder, neck, and lumbar spine osteoarthritic degeneration).”
Why is this important? Because we know that adjacent segment disease is common in spinal fusion patients. This means that the forces that should be absorbed by the SI joint are instead shunted to other joints which become overloaded and arthritic due to the extra wear and tear. The next joints up from the SI joint are the lumbar spine and the next joint down is the hip. Hence any degenerative arthritis that occurs in either location would be due to the SI fusion until proven otherwise. To learn more, see my video below:
FDA Reports of Complications
This week, I was searching for the latest studies on the SI Bone iFuse implant and instead, I ran across the FDA report of a serious adverse event. My interest was piqued, so I located the FDA device reporting system and I then found 500 complication hits on the iFuse Implant System! So let’s dig in.
These 500 incidents span three years (2016-2019) or about 14 reports a month for 36 months. These are VOLUNTARY reports, so they clearly represent under-reporting of any complications sustained by patients getting the procedure. How much more is out there is unknown, but here’s what the FDA reports say based on the type of complication:
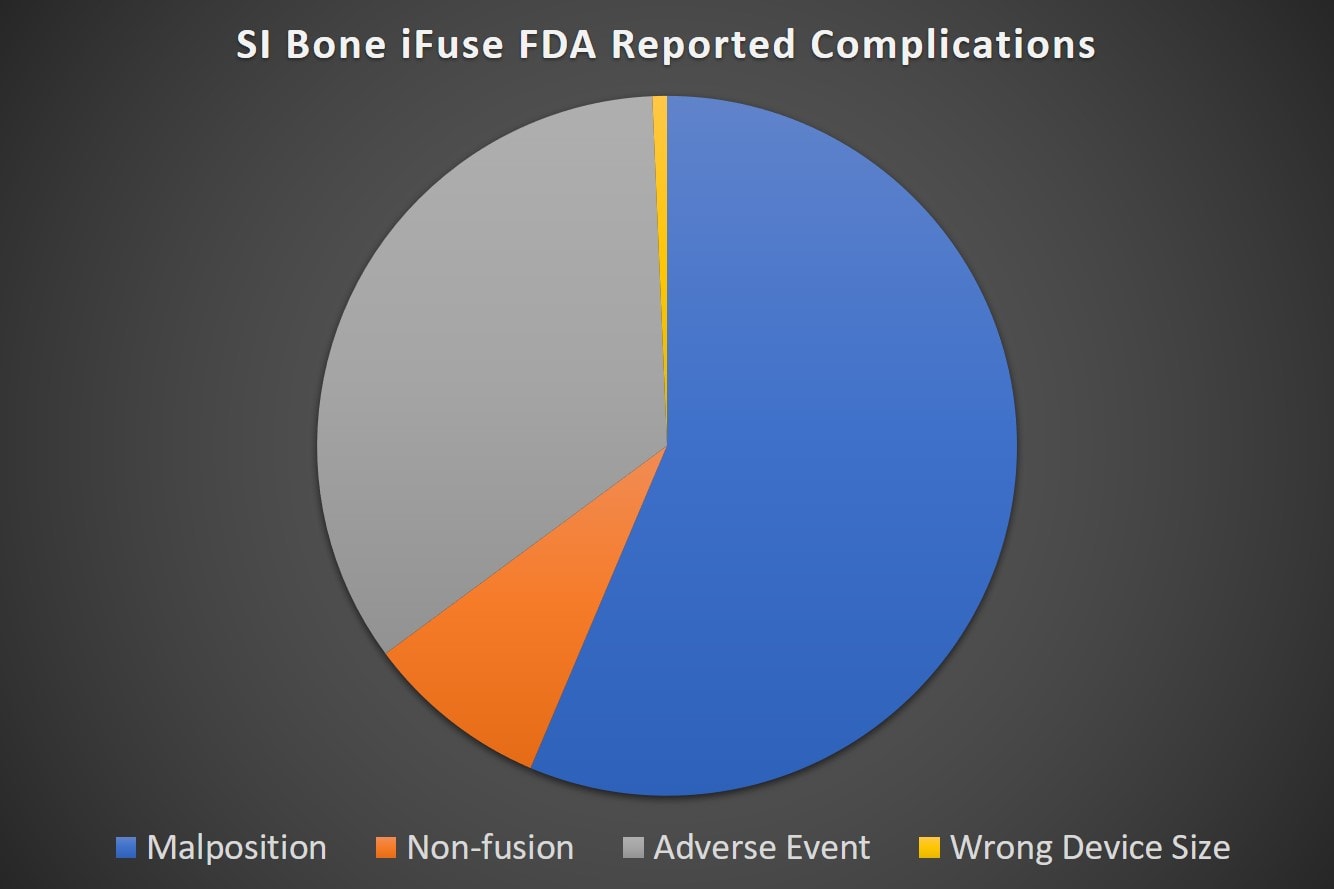
Note that the single most common complication type was placing the device in the wrong spot. This caused all sorts of issues like the device banging into and compressing nerves, increased pain, or device loosening. The adverse event category has all sorts of things like infections, increased pain for unknown reasons. failure to fuse, etc… The non-fusion is what it sounds like, the joint failed to grow together.
Since the report has 500 entries in a spreadsheet (available here for download) and each one of those lines has a detailed description of what went wrong, I created a word cloud of the most frequent medically meaningful terms (the bigger the word here the more frequently it was mentioned):

The most concerning words/phrases: removal of the device, pain, terms that indicate that the surgeon damaged or hit a spinal nerve, and terms about placing this large implant in the wrong spot. My guess is that an extensive discussion of these risks with the patients considering this surgery would likely dissuade a significant number from going forward.
What If They Have to Remove This Device?
Since the surgeon is drilling a couple of holes through the SI joint, this procedure is a one-way street. Meaning if the joint doesn’t fuse and the hardware has to be removed and isn’t replaced by something else, the joint has been destroyed and is still unstable. Hence, in my medical opinion, that’s a bad day for everyone.
Are There Less Invasive Options for Treating SI Joint Instability?
It’s really tough to see patients getting these really invasive one-way fusions when in my personal decades-long experience 90% of these patients could easily avoid this by using their own body to heal these damaged or loose SI joint ligaments. Realize that I’m not talking about a steroid injection into the SI joint to reduce swelling and pain in the short-term (which is done as a diagnostic block for the iFuse procedure), but instead, injections that are capable of healing tissues long-term. Let’s review.
How does that work? For most patients, it means getting a precise image-guided injection of their own platelet-rich plasma (PRP) into the ligaments and joint (6). Prolotherapy also works as well (7). If those approaches don’t work, then getting your own bone marrow concentrate (BMC or a same-day stem cell procedure) injected usually gets it done.
How could these regenerative injection procedures work? By promoting the damaged ligaments to beef up and tighten. In addition, both of those procedures are much less invasive than an iFuse implant and far less costly. However, disturbingly, very few patients who end up with an irreversible SI Bone implant get offered these latest and more elegant options. Why? iFuse implants are simply more profitable.
The upshot? I am NOT a fan of this device or approach to treating SI joint instability. This is a maximally invasive way to treat what can usually be solved using true image-guided minimally invasive ligament injection. The fact that there is an FDA database chock filled with serious complications from using this device is not at all surprising. I guess I’m only surprised that so many patients are signing up for this procedure given the risks!
____________________________________
References:
(1) Duhon BS, Cher DJ, Wine KD, Kovalsky DA, Lockstadt H; SIFI Study Group. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: A Prospective Study. Global Spine J. 2016;6(3):257–269. doi:10.1055/s-0035-1562912
(2) Duhon BS, Bitan F, Lockstadt H, et al. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: 2-Year Follow-Up from a Prospective Multicenter Trial. Int J Spine Surg. 2016;10:13. Published 2016 Apr 20. doi:10.14444/3013
(3) Whang P, Cher D, Polly D, et al. Sacroiliac Joint Fusion Using Triangular Titanium Implants vs. Non-Surgical Management: Six-Month Outcomes from a Prospective Randomized Controlled Trial. Int J Spine Surg. 2015;9:6. Published 2015 Mar 5. doi:10.14444/2006
(4) Darr E, Cher D. Four-year outcomes after minimally invasive transiliac sacroiliac joint fusion with triangular titanium implants. Med Devices (Auckl). 2018;11:287–289. Published 2018 Aug 29. doi:10.2147/MDER.S179003
(5) Schoell, K., et al., Postoperative complications in patients undergoing minimally invasive sacroiliac fusion. Spine J. 2016 Nov;16(11):1324-1332. doi: 10.1016/j.spinee.2016.06.016.
(6) Singla V, Batra YK, Bharti N, Goni VG, Marwaha N. Steroid vs. Platelet-Rich Plasma in Ultrasound-Guided Sacroiliac Joint Injection for Chronic Low Back Pain. Pain Pract. 2017 Jul;17(6):782-791. doi: 10.1111/papr.12526.
(7) Kim WM, Lee HG, Jeong CW, Kim CM, Yoon MH. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010 Dec;16(12):1285-90. doi: 10.1089/acm.2010.0031.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.