What Is a Mitlet?
Credit Platelet Image: Shutterstock
Just when you thought you knew everything there was to know about platelets and PRP, something new crops up. That’s why I love medicine, as we’re just starting to know what we don’t know. Let’s explore a new idea, called “Mitlets”.
What the Heck is a Mitlet?
If you read this blog, you’ve heard quite a bit about platelets. These are the little guys that clot your blood and contain things called alpha vesicles that are packed with growth factors. These are what help to heal or regenerate tissues.
Platelets also emit exosomes, which contain all sorts of things (7-9). These are small packets called vesicles that usually contain snippets of mRNA which can make new proteins. These proteins can often be used to help build new tissue.
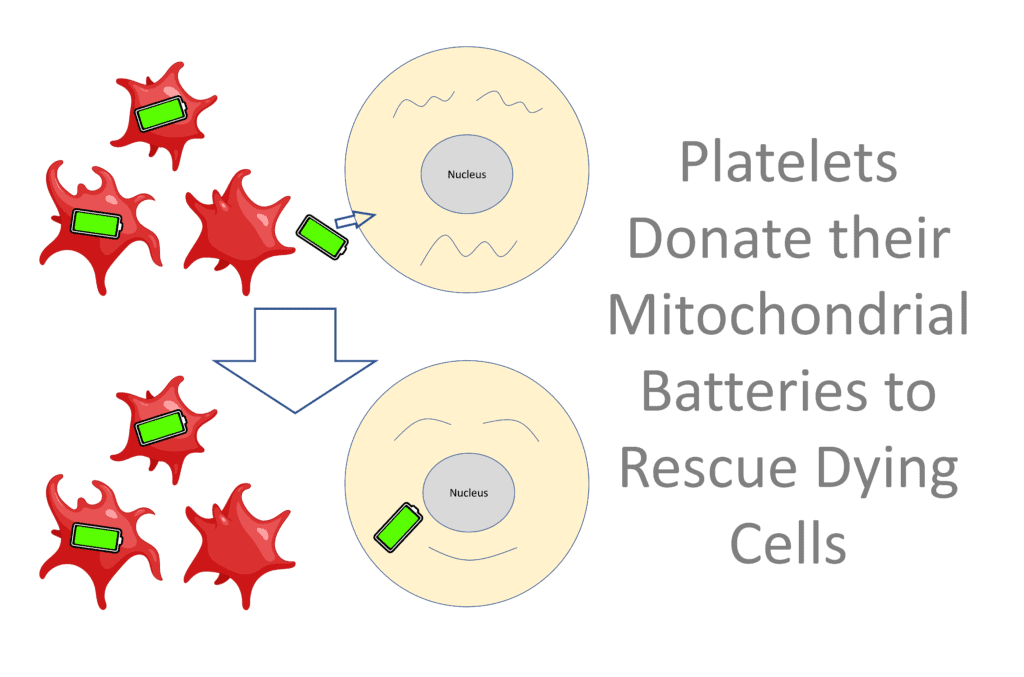
However, recent research shows that platelets also give off vesicles that contain mitochondria (1). These newly discovered platelet-derived vesicles are called “Mitlets”. Why is this important? Let’s dig in.
Rebooting Dying Cells
Mitochondria are like a Tesla battery pack for cells in that they supply the energy for everything that happens. When cells die, one way that happens is that the mitochondrial battery pack gives out.
One of the ways that stem cells repair dying cells is by giving their mitochondria to cells with bad battery packs (2). It’s also been shown in the lab that adding mitochondria to stem cells enhances their repair capabilities (3). We also know that this happens in the body as well (4).
Can platelets do this? Yep. Platelets can transfer their mitochondrial battery packs to mesenchymal stem cells, enhancing their wound healing capability( 5).
In fact, this whole idea of cells donating their mitochondria to other cells as a means of tissue repair is now exploding (6). It turns out that this has been reported in the central nervous system, heart and cardiovascular system, and in the lungs. It also goes the other way, with platelet mitochondria causing too much inflammation in disease states like Lupus.
Commercializing Mitlets
One group out there is taking expired platelets and finding ways to isolate the Mitlets and/or the exosomes containing mitochondrial battery packs. The idea is that one day, patients in need of regeneration will be able to get a Mitlet injection. However, clinical trials have yet to begin.
The upshot? If you want your dying cells to get a mitochondrial battery boost, the best way is through platelet-rich plasma and/or treatments containing mesenchymal stem cells. In addition, this area of research may change how we assay whether or not we have enough “oomph” behind our PRP or stem cell containing treatments. Meaning, in addition to how many growth factors or exosomes they contribute, it may be critical to know how often they can rescue other cells by donating their mitochondrial batteries!
_______________________________________
References:
(1) Melki I, Tessandier N, Zufferey A, Boilard E. Platelet microvesicles in health and disease. Platelets. 2017 May;28(3):214-221. doi: 10.1080/09537104.2016.1265924. Epub 2017 Jan 19. PMID: 28102737.
(2) Jiang, D., Gao, F., Zhang, Y. et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis 7, e2467 (2016). https://doi.org/10.1038/cddis.2016.358
(3) Guo, Y., Chi, X., Wang, Y. et al. Mitochondria transfer enhances proliferation, migration, and osteogenic differentiation of bone marrow mesenchymal stem cell and promotes bone defect healing. Stem Cell Res Ther 11, 245 (2020). https://doi.org/10.1186/s13287-020-01704-9
(4) Vallabhaneni KC, Haller H, Dumler I. Vascular smooth muscle cells initiate proliferation of mesenchymal stem cells by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2012;21(17):3104-3113. doi:10.1089/scd.2011.0691
(5) Levoux J, Prola A, Lafuste P, Gervais M, Chevallier N, Koumaiha Z, Kefi K, Braud L, Schmitt A, Yacia A, Schirmann A, Hersant B, Sid-Ahmed M, Ben Larbi S, Komrskova K, Rohlena J, Relaix F, Neuzil J, Rodriguez AM. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021 Feb 2;33(2):283-299.e9. doi: 10.1016/j.cmet.2020.12.006. Epub 2021 Jan 4. Erratum in: Cell Metab. 2021 Mar 2;33(3):688-690. PMID: 33400911.
(6) Liu, D., Gao, Y., Liu, J. et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Sig Transduct Target Ther 6, 65 (2021). https://doi.org/10.1038/s41392-020-00440-z
(7) Parsons MEM1, Szklanna PB1, Guerrero JA, et. al. Platelet Releasate Proteome Profiling Reveals a Core Set of Proteins with Low Variance between Healthy Adults. Proteomics. 2018 Aug;18(15):e1800219. doi: 10.1002/pmic.201800219.
(8) Walsh TG, Poole AW. Do platelets promote cardiac recovery after myocardial infarction: roles beyond occlusive ischemic damage. Am J Physiol Heart Circ Physiol. 2018;314(5):H1043–H1048. doi: 10.1152/ajpheart.00134.2018
(9) Preußer C, Hung LH, Schneider T, et al. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Vesicles. 2018;7(1):1424473. Published 2018 Jan 15. doi: 10.1080/20013078.2018.1424473

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
