Why Is Regenexx the Best That’s Out There?
How can Regenexx claim that it’s the most advanced and best-researched orthopedic stem cell therapy in the world? After all, if you cruise the Internet, you’ll find any number of clinics, all proclaiming that they’re the “best” or the “greatest” or “experts.” However, if you look “under the hood” of any of these web-sites and then compare that to what you’ll read below, you’ll see it’s like comparing a subcompact car to a Ferrari. Some of what you’ll read is at a more complex and scientific level than my usual blog style, but that’s really necessary to hit this one out of the park.
Background: The doctors who founded Regenexx (Christopher Centeno and John Schultz) were the first in the U.S. to begin to use stem cells to treat orthopedic conditions. Since then, these physicians have created a network of highly select and highly trained physicians who claim to be performing the most advanced and best-researched stem cell procedures in the U.S. and the world. What evidence is there that supports these claims?
How Does Regenexx Compare to the State of Current Sophistication and Research?
At issue is the application of same-day stem cell treatments (bone marrow concentrate, or BMC) and platelet rich plasma (PRP) to treat orthopedic applications via image-guided injection-based treatment.
What is Regenexx? We are a group of 30-plus U.S. medical practices that use the same BMC and platelet-treatment protocols. We all fund a university-style clinical-research and lab-research facility headquartered in Colorado. Given that much of the data reported below has been gathered via either our registry or the university-style lab, a short discussion about both is warranted.
What is the Regenexx registry? We use university-based, open-source clinical research organization software (Clinovo) and have four full-time employees who track outcomes and complications. Preoperative data is collected on all BMC-treated patients via validated pain/functional questionnaires. The registry sends questionnaires at 1, 3, 6, and 12 months and then annually on an ongoing basis. The software sends automated e-mails two times, and if the patient doesn’t respond, then up to three phone calls are initiated. If the patient fails to respond, that outcome end point is lost to follow-up and the patient is reacquired at the next time point. This registry has now been moved into a 501(c)(3) nonprofit—The Interventional Orthopedics Foundation.
What is the Regenexx research lab? In Colorado we have three full-time scientists and a lab equipped with normoxic and hypoxic incubation, flow cytometry, fluorescence-activated cell sorting, inversion microscopy, fluorescent microscopy, PCR LightCycler (a rapid high-throughput, plate-based, real-time PCR amplification and detection instrument), ELISA, multiplex microarray ELISA, -80 and -150 cryostorage, and other technologies. We conduct basic in-vitro experiments to improve our procedures at this privately funded lab. You’ll see many examples below of how that works. Please 
What does all of this compare to? Right now, physicians offering FDA-compliant bone-marrow-based stem cell therapies fall into one of two categories: they use an automated bedside centrifuge or they use an in-house laminar flow hood to process samples on site. For both types of practices, there is no clinical or in-vitro research being conducted to continuously improve the procedures. Both types of practices use simple technology that’s fixed in what it can produce. While I’ll discuss further the issues this causes and how the Regenexx group practice has pushed beyond these technologies, it’s important to realize that when we claim to be more advanced, “top,” “world’s leading,” and better researched, these are supported by the world’s largest clinical registry of its kind and one of the most sophisticated private-practice-based lab-research operations in the world.
The substantiation for these claims can be broken into key components. To understand this, it’s helpful to review how an autologous bone marrow concentrate injection is used to treat orthopedic conditions.
This can be further broken down into the following:
- The bone marrow aspiration (BMA)
- The processing of the bone marrow aspirate sample
- The reinjection
- Ongoing research
After reviewing the procedure, I will then focus on how Regenexx is superior to what other physicians are offering.
The Bone Marrow Aspiration
Regenexx as a group medical practice, as of the spring of 2016, has performed approximately 4,000 bone marrow aspirations (BMAs) since 2005 and uses only advanced techniques to perform this procedure. First, what is a BMA? Second, why is it important to quantify cell dose, and how does that intersect with how the BMA is performed? Finally, why is it critical to perform the procedure using imaging guidance? As you will see, how we approach a BMA makes us the consistently most advanced purveyors of the BMA technique.
A bone marrow aspiration (BMA) is performed by using a trocar to cannulate the bone, commonly in the pelvis. The liquid portion of the bone marrow (termed an aspirate) is then removed via syringe. The typical way this procedure is performed (single site and high volume with no knowledge of dose) is quite different from how it’s performed within the Regenexx group medical practice.
The most common method of obtaining a BMA is the use of a trocar to cannulate one site, commonly in the pelvis, where 60 ml is drawn into a syringe. However, as you’ll see, our own research and that published by others has shown that this is not the best way to maximize stem cell yield from the BMA. At Regenexx, our technique involves cannulating multiple sites with lower-volume draws.
First, how important is it to maximize mesenchymal stem cell (MSC) content in a BMA? In 2015 we published a dosing paper that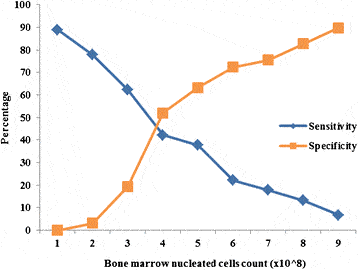
Others have reported similar results in different applications. As an example, a recent small case series published on the use of intradiscal treatment with BMC found a direct correlation between colony-forming units (an indirect measure of MSC content) and outcome. In addition, the efficacy of BMC when used for bone-healing applications, such as nonunion and osteonecrosis, is also dependent on MSC dose. Hence, the MSC content of BMC or proxies for it are directly related to clinical outcome in a classic direct dose-response relationship.
Based on this dose-response relationship, one of the differentiators that’s important here between Regenexx and everyone else performing this technique becomes apparent. Ninety-nine percent of the physicians who use BMC have no ability to count the cell number they’re harvesting. This is because the automated bedside centrifuges don’t provide this count, and the cell-counting devices that are available require trained lab staff and validation, which aren’t possible in the average practice. However, all Regenexx group practices count the number of cells available to determine dose.
Think about this for a moment. The current state of the art at practices worldwide that use BMC is to inject an unknown dose. There is obviously no other area of medicine where a substance is injected into the body at an unknown dose or concentration.
If dose is important and only Regenexx physicians routinely determine dose, how important is it to maximize MSC content (dose) during the BMA technique? If we use the data we have collected on minimum knee OA dose (400M TNCC) and the data we have amassed in counting 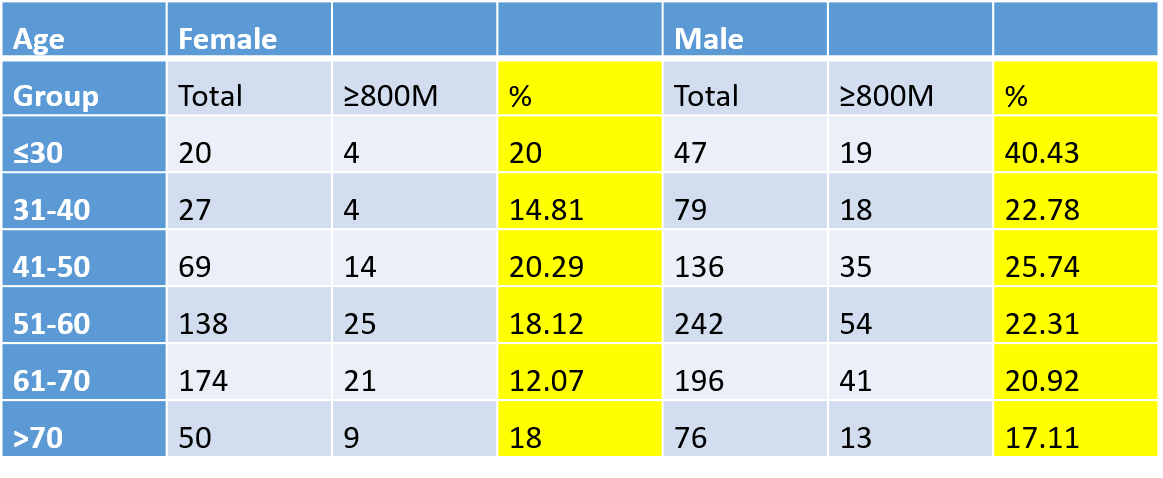
Hence, the percentage of patients who can achieve 800M cells and comfortably treat their bilateral knees is small. Given that this is a common request and almost no clinics using BMC have the ability to count cells, many are likely treating this condition with far too few cells. At the very least, this illustrates the need to maximize the number of MSCs that can be obtained via a BMA.
We have known, based on the peer-reviewed literature since the late ’90s, that cannulating many bone marrow sites and aspirating small volumes increase MSC yield over a single site cannulated to withdraw a larger volume (see article 1, article 2, article 3). As discussed above, our experience in speaking with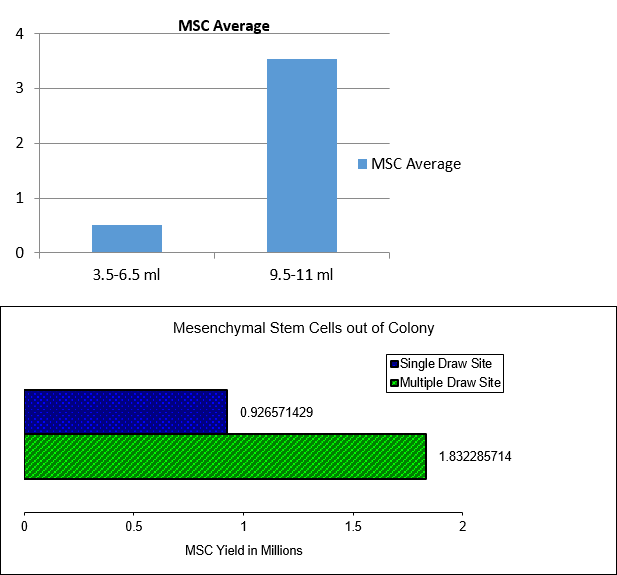
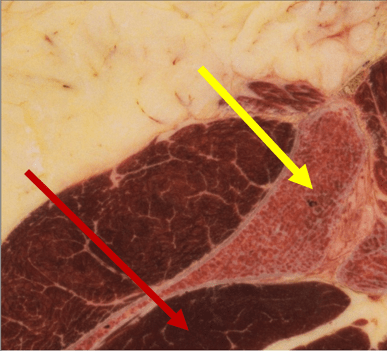
Given that our national team is in constant communication with hundreds of physicians who are prospective candidates to join our group practice, and most commonly these things are not done, these are the differentiators for “most advanced” in the area of BMA:
- All Regenexx doctors perform BMAs in the way the peer-reviewed literature and the internal research performed by the practice shows maximizes MSC yield
- All Regenexx doctors count cells and know dose
- All Regenexx doctors use imaging guidance to harvest cells
The Processing of the Bone Marrow Aspirate Sample
Almost all physicians who use bone marrow concentrate utilize automated bedside centrifuges to process the bone marrow aspirate sample. The goal of this processing is to concentrate the stem cell containing fraction of the aspirate. The dose discussion above is one of the reasons that many physicians and scientists believe this concentration is needed.
All automated machines use the same basic process to concentrate BMC—they capture the “buffy coat” with varying levels of efficiency. This is the small, gray in color, middle layer that appears after BMC has been centrifuged—the traditional source of MSCs derived from bone marrow. Given changes in hydration and hematocrit, it’s not hard to see that this layer may appear at differing heights in different patients. Most of the automated bedside machines in use today account for this interpatient variation by taking a large cut of sample above and below the buffy coat, reducing the total BMA volume from 60 cc to approximately 10 cc. Given that the size of the buffy coat in a 60 ml sample is usually 1–2 cc, this creates a concentration problem (i.e., 1–2 cc of active ingredient diluted in 10 cc). When the Regenexx group medical practice first began, our focus was also on capturing this layer. However, manually processing this layer in a laminar flow hood in a medical practice resulted in much higher concentrations as the usual dilution we could accomplish was 1–2 ml of active buffy coat in approximately 3 ml of total BMC. Hence, the first version of our process was more advanced than automated bedside units because it produced an injectate with 3–4 times the concentration of the buffy-coat active ingredient over automated bedside systems.
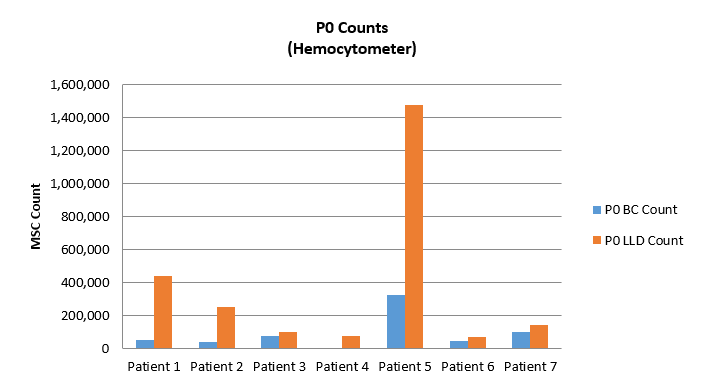
The chart entitled “PO Counts” to the left is a comparison of the MSC content of the two layers with the traditional buffy-coat (BC) isolation versus the new source (we call this LLD). As you can see, the differences in the number of MSCs obtained via colony formation after plating the buffy coat versus the LLD is stark. In all seven patients tested, LLD MSC content significantly beats the buffy coat.
In addition, other studies performed in our lab found that the MSCs in the LLD were faster growing and more chondrogenic (more capable of cartilage repair) than those found in the buffy coat. For example, the graph entitled “Day 8 mRNA Levels” to the right shows that the gene expression panel associated with chondrogenesis is hugely upregulated in LLD cells versus buffy-coat cells.
When we added these two fractions together, we produced a BMC that had an MSC content that was reliably 5–20 times higher than what could be obtained via automated bedside centrifuges. In addition, given the orthopedic-injury focus of the group medical practice, the fact that MSCs from the LLD are present is important to us. However, we also sought additional validation that this new processing technique would translate into better clinical success. The graph entitled, “Second Generation Regenexx-SD Procedure” represents a prospective registry comparison trial between the first-generation processing and the newer 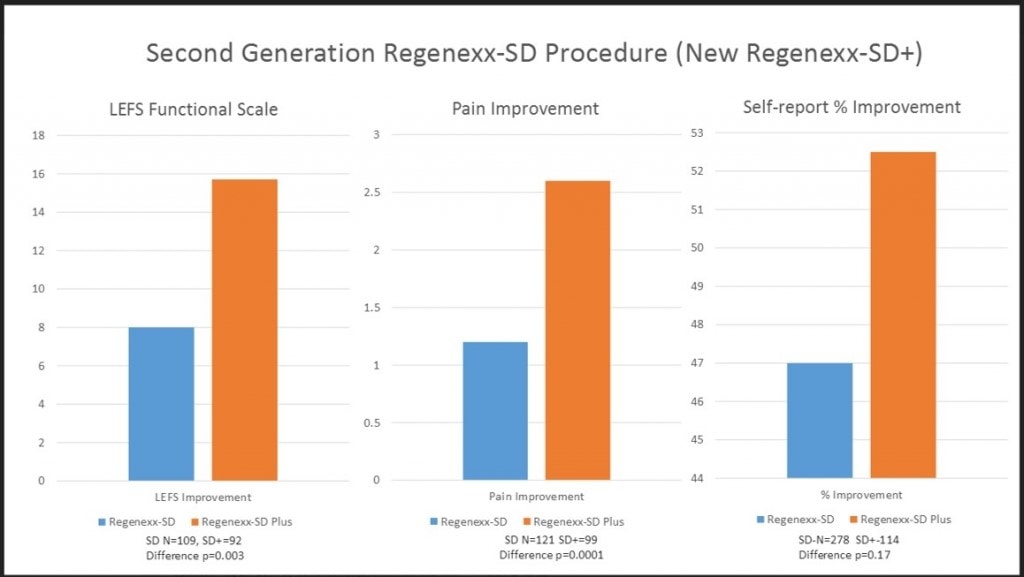
What’s Different About Regenexx PRP?
Another common practice with the use of BMC to treat osteoarthritis is to add platelet rich plasma (PRP) to the injection. The goal of adding PRP to BMC is that as the platelets degranulate, they will release growth factors helpful to MSCs. Regenexx has also conducted its own in-vitro research to optimize this process. We first conducted experiments looking at the differences between red/white blood-cell-rich PRP (RBC+/WBC+) versus red/white blood-cell-poor PRP (RBC-/WBC-).
The graph to the right contains the results of an experiment that looked at the ability of the two different types 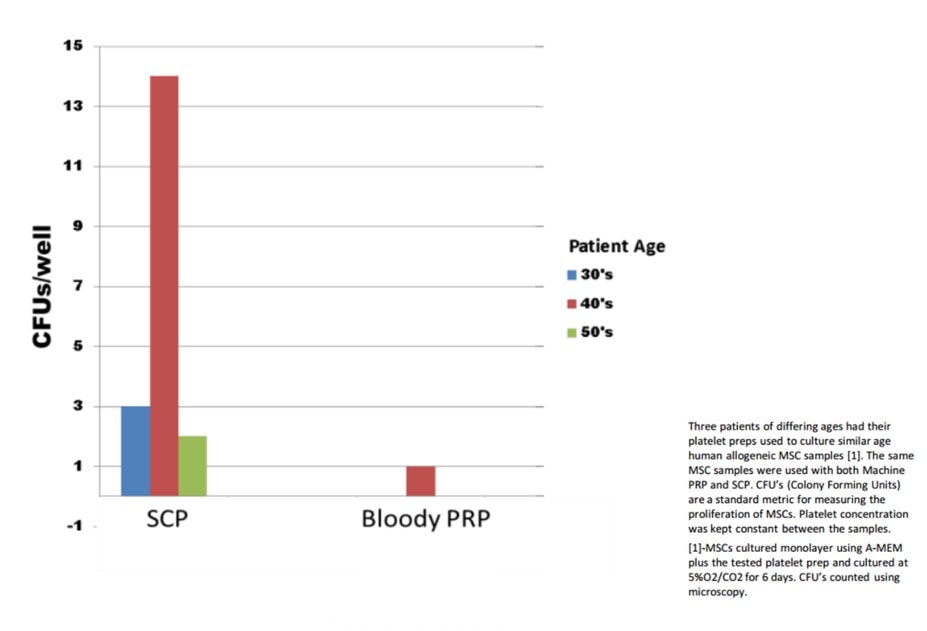
The other variable in using PRP to support MSC growth in BMC is platelet concentration. Most bedside units that produce PRP can only muster a concentration of 3–7 times over baseline. Is this enough? We have also tested this in-vitro.
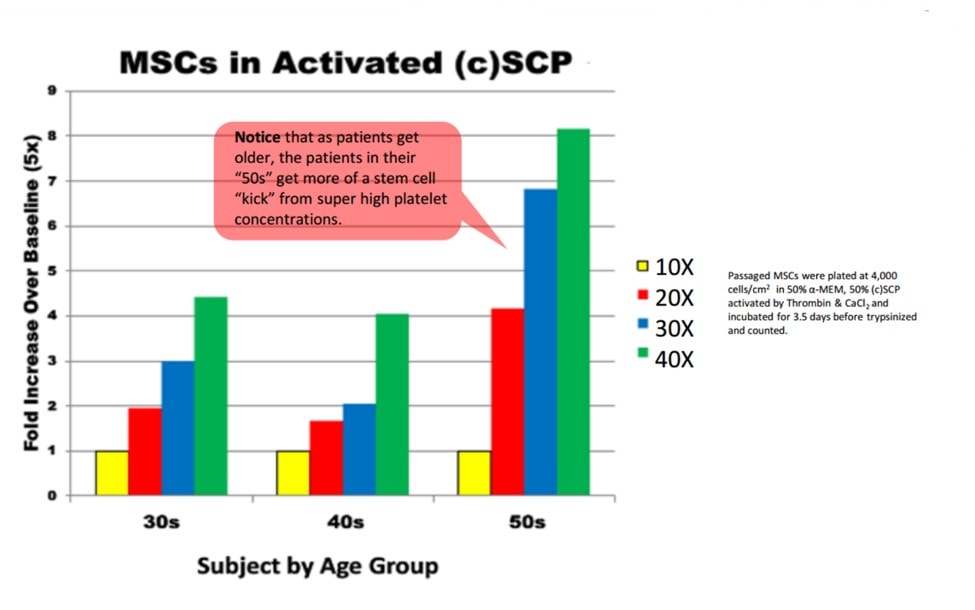
How Regenexx Uses a Unique Blood Product—Platelet Lysate
There’s another unique component to the procedures used by our medical group not used by others—platelet lysate (PL). PL is created by the lysis of platelet rich plasma, which releases growth factors (GFs) contained in platelets into the serum. So while platelets in PRP slowly degranulate their GFs over approximately one week, platelet lysate has all of these GFs immediately available. Hence PRP is rarely used to provide GFs to culture MSCs in-vitro, while PL is commonly used for this purpose. Regrettably, there is no automated bedside centrifuge that can be purchased that creates PL; hence, its clinical use is limited to those who have established small office-based lab facilities.
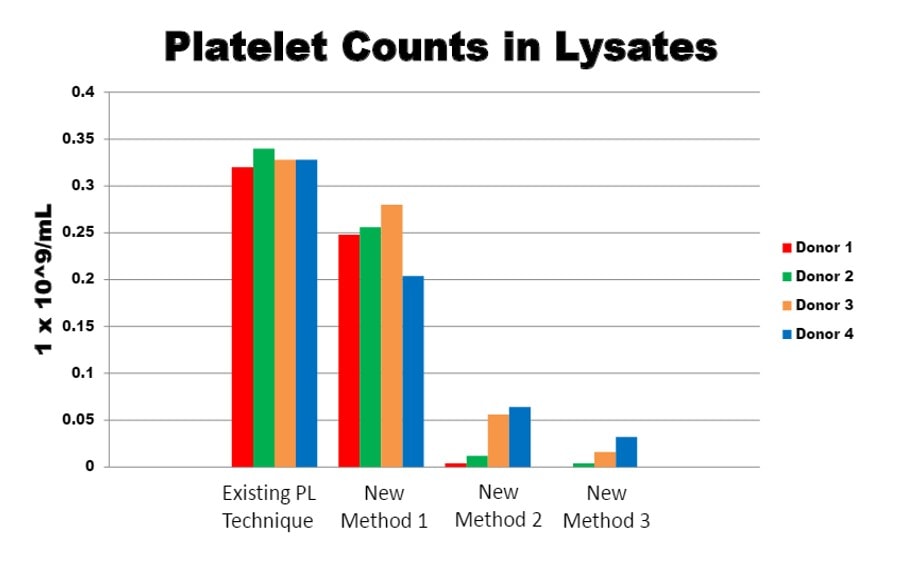
We next studied the growth factor content produced by the various lysis methods. The graphs below represent internal data on ELISAs that demonstrate GF levels in the first (PL) and second generation (PLM).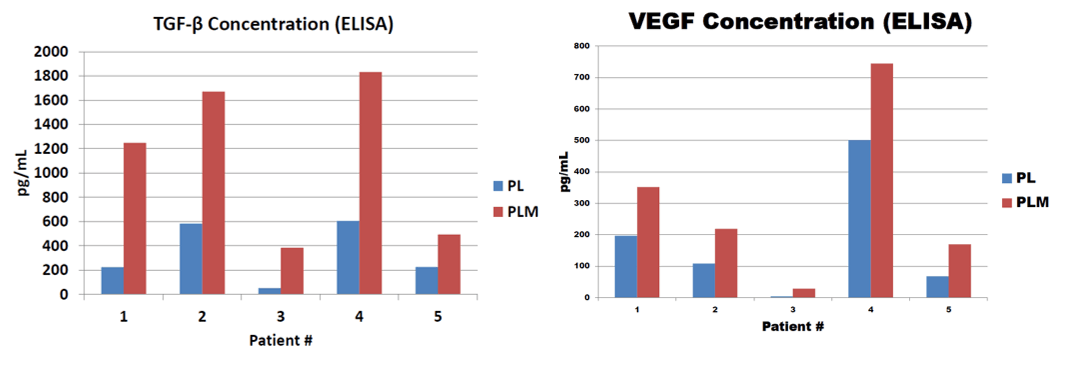
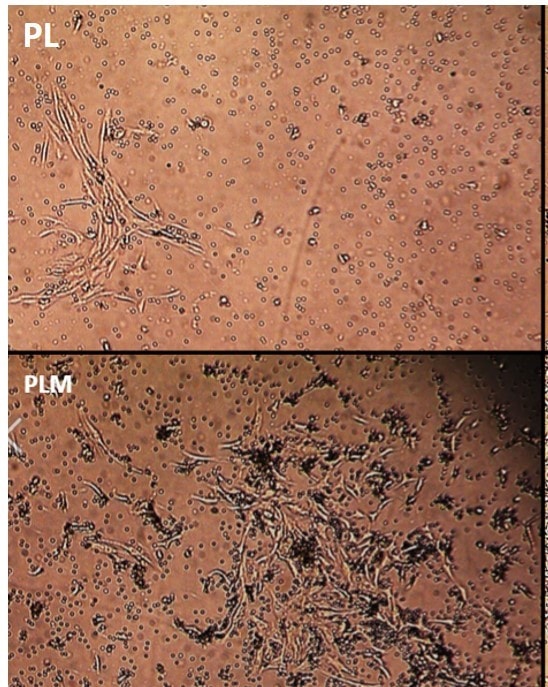
In summary, these are the differentiators between the automated bedside centrifuges used by most practices and Regenexx that support our claims:
- Manual processing allows much higher concentrations of the buffy-coat active ingredient
- Adding in LLD cells allows still higher concentrations of MSCs, and this, based on our internal research, has shown better clinical outcomes in our most commonly treated patient type (knee OA)
- Using RBC-/WBC- PRP at very high concentrations allows for better in-vitro evidence of MSC support than lower-concentration RBC+/WBC+ PRP
- The use of a proprietary high growth factor platelet lysate that shows significantly improved in-vitro MSC proliferation
The Reinjection
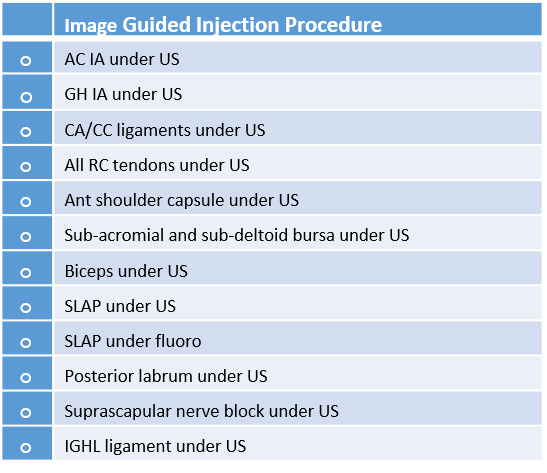
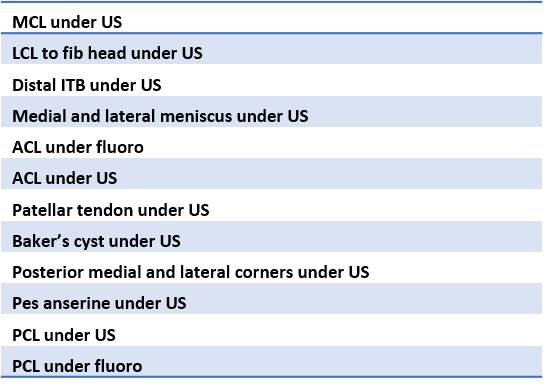
Given that we have physicians who apply to be a part of our group medical practice, we use these core-skills lists to determine if a physician has the base training to be able to perform our procedures. The majority of physicians who report their existing skills back to us don’t have the ability to perform these procedures. However, for a provider in the Regenexx group medical practice to advertise that he or she has these skills in a given area, the provider needs to demonstrate competency in the entire array of imaging-guided procedures by body area during a cadaver course to an instructor. As a result, we accept only about 10% or less of the medical providers who want to join our group.
How does this compare to the average provider offering stem cells and PRP? As discussed, most don’t use guidance at all, instead performing blind procedures. While some do use ultrasound guidance and a few use fluoroscopy (real time x-ray imaging), almost none have both technologies available. In addition, even when these imaging guidance technologies are available, the vast majority of providers only know how to perform the simple intra-articular injections on these lists. Very, very few understand how to perform the more advanced procedures.
As an example, the provider might understand how to inject a knee and get the cells inside the joint using ultrasound. However, he or she doesn’t know how to inject the meniscus, ACL, PCL, MCL, or LCL. Or the provider might know how to inject the shoulder and get the cells generally inside the joint, but can’t inject the superior labrum or rotator cuff.
How One Procedure out of Twelve Required Knee-Injection Skills Supports the Thesis of “Most Advanced”
Let’s move from a 30,000-foot overview of the skills needed to be in our group practice to a “rubber meets the road” view on one specific knee procedure on that list. I’ll first review the data we have published and are collecting on this technique and then an exemplar of the results. Finally, I’ll discuss the specifics of this method.
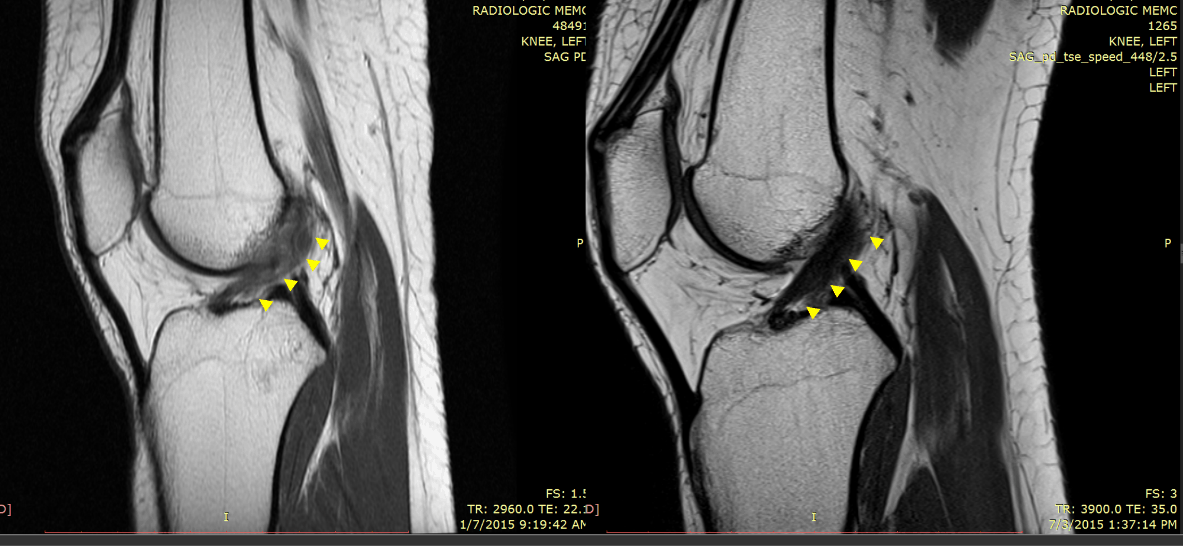
One of the indications where we have seen great success is knee anterior cruciate ligament (ACL) tears. As an example, our recently published case series showed MRI evidence of the ability of our injection-based BMC protocol pioneered by our practice to heal partial and complete nonretracted ACL tears.
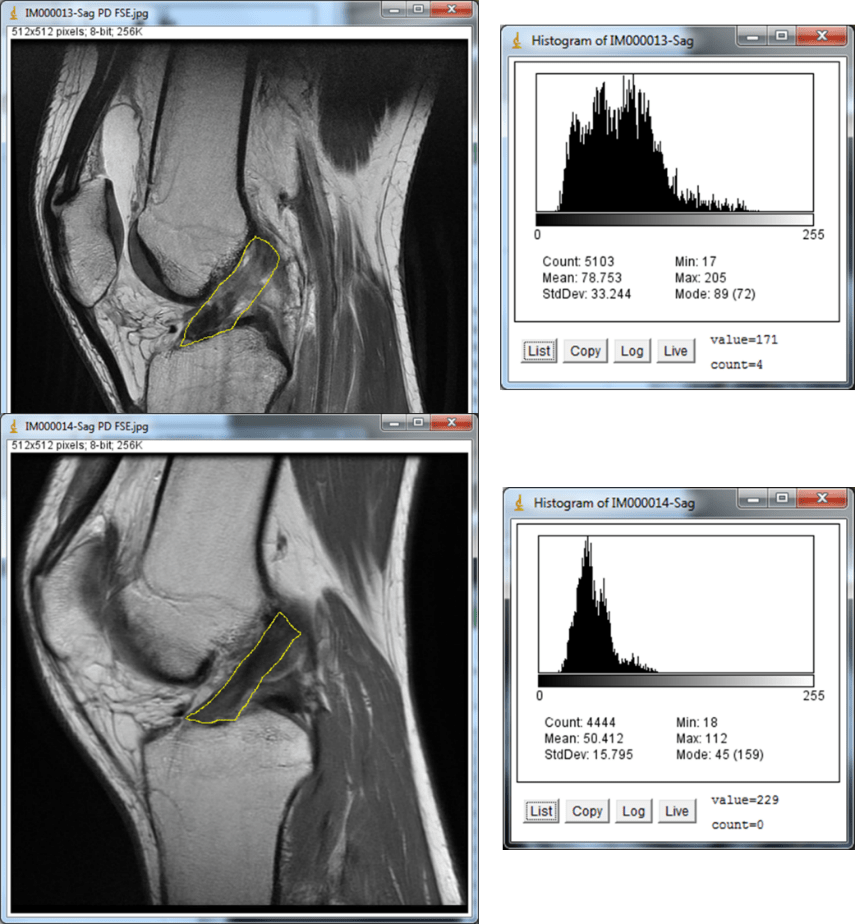
Below is an MRI exemplar of the results of this procedure. This is a patient with a full-thickness ACL tear who was told she needed ACL reconstruction, but she opted for precise placement of BMC into the remaining disrupted ACL fibers. On the left is the immediate preinjection film, and on the right is the three-month post MRI. The before image was read out by the reading radiologist as a complete ACL tear, and the after image as a “normal ACL.”
We are now readying a second, larger case series for publication and have self-funded a randomized controlled trial (RCT). Note that no physician who wanted to join our network (who would likely be a typical cross section of U.S. physicians performing BMC and PRP injections) knew how to perform this procedure before joining the network. Let’s delve deeper into that technique.
In the case of the ACL procedure pioneered by our medical group, BMC is seeded into the ACL at the origin and insertion of each bundle (posteolateral and anteromedial) under C-arm fluoroscopy. This requires significant technical skill and training. This is not a procedure that is taught in residency programs, courses, or private practices. It is only used currently by physicians trained by our group practice. We have many such procedures.
Hence, in support of the “most advanced” as far as cell placement we have the following:
- Injections are only performed with guidance versus the more common blind variety.
- The vast majority of physicians do not have these skills as demonstrated by our own data.
- Many of the procedures we offer are unique to our medical group and have been pioneered by us and are not widely taught.
Ongoing Research
Our group practice has been involved in ongoing research to improve our procedures. This can be broken down to the following:
- Registry data collection and published data sets
- Ongoing clinical and lab research
Registry Data Collection
We have collected outcome and complications data in a registry since 2005. This effort has cost millions of dollars to administer. As of today’s date, we are tracking more than 4,000 stem cell treated patients. The registry in 2015 was turned over to a 501(c)(3) nonprofit—The Interventional Orthopedics Foundation (IOF). The IOF is now running the day-to-day operations of the registry.
The data from this registry has been the subject of many different papers published in the peer-reviewed literature:
BMC Musculoskeletal Disorders 2015, 16:258
Background: Prior studies describing the treatment of symptomatic knee osteoarthritis with injections of bone marrow concentrate have provided encouraging results. The relationship between the cellular dose contained within the bone marrow concentrate and efficacy of the treatment, however, is unclear. In the present study we describe clinical outcomes for symptomatic knee osteoarthritis in relation to higher and lower cell concentrations contained within a bone marrow concentrate treatment protocol.
Methods: Data from an ongoing patient registry was culled to identify 373 patients that received bone marrow concentrate injections for the treatment of 424 osteoarthritic knee joints. The clinical scales for these patients were assessed at baseline and then tracked post-procedure at 1, 3, 6 and 12 months, and annually thereafter. Tracked outcomes included the numeric pain scale; a lower extremity functional questionnaire; an International Knee Documentation Committee scale; and a subjective improvement rating scale. Using pain and functional outcome measures, a receiver operating characteristic analysis was used to define an optimal clinical outcome threshold at which bone marrow nucleated cell count could be divided into either a lower or higher cell count group within a treatment protocol.
Results: The lower and higher cell count groups were defined using a threshold of 4 × 10 8 cells. There were 224 and 185 knee joints treated in the lower (≤4 × 10 8 ) and higher (>4 × 10 8 ) cell count groups respectively. Most joints were diagnosed with early stage knee osteoarthritis. Both the lower and higher cell count groups demonstrated significant positive results with the treatment for all of the pain and functional metrics. The higher cell count group reported lower post treatment numeric pain scale values, in comparison with the lower cell count group (1.6 vs. 3.2; P < 0.001). No significant differences were detected for the other metrics, however.
Conclusions: Improved function and reduced pain was observed in patients treated with a bone marrow concentrate protocol regardless of cellular dose; however, patients receiving a higher concentration of cells reported a better pain outcome in comparison with the lower dose group. These preliminary findings suggest that cell dose may be an important factor governing clinical outcomes in autologous bone marrow concentrate treatment of knee osteoarthritis. Further studies using a larger patient population may help elucidate these findings.
Journal of Pain Research 2015, 8:437–447
Introduction: This was a prospective case series designed to investigate treatment for anterior cruciate ligament (ACL) tears using an injection of autologous bone marrow concentrate.
Methods: Consecutive adult patients presenting to a private outpatient interventional musculoskeletal and pain practice with knee pain, ACL laxity on exam, and magnetic resonance imaging (MRI) evidence of a grade 1, 2, or 3 ACL tears with less than 1 cm retraction were eligible for this study. Eligible patients were treated with an intraligamentous injection of autologous bone marrow concentrate, using fluoroscopic guidance. Pre- and postprocedural sagittal MRI images of the ACLs were analyzed using ImageJ software to objectively quantify changes between pre- and posttreatment scans. Five different types of measurement of ACL pixel intensity were examined as a proxy for ligament integrity. In addition pain visual analog scale (VAS) and Lower Extremity Functional Scale (LEFS) values were recorded at baseline and at 1 month, 3 months, 6 months, and annually postinjection. Objective outcomes measured were pre- to post-MRI measurement changes, as analyzed by the ImageJ software. Subjective outcomes measured were changes in the VAS and LEFS, and a self-rated percentage improvement.
Results: Seven of ten patients showed improvement in at least four of five objective measures of ACL integrity in their postprocedure MRIs. In the entire study group, the mean gray value, median, raw integrated density, and modal gray value all decreased toward low-signal ACLs (P=0.01, P=0.02, P=0.002, and P=0.08), indications of improved ligament integrity. Seven of ten patients responded to the self-rated metrics follow up. The mean VAS change was a decrease of 1.7 (P=0.25), the mean LEFS change was an increase of 23.3 (P=0.03), and mean reported improvement was 86.7%.
Conclusion: Based on this small case series, autologous bone marrow concentrate shows promise in the treatment of grade 1, 2, and possibly grade 3 ACL tears without retraction. Further investigation using a controlled study design is warranted.
Journal of Pain Research 2015, 8:269–276
Introduction: Shoulder pain is a common musculoskeletal complaint in the general population. Bone marrow concentrate (BMC) injections offer promising potential as a minimally invasive approach for treatment of shoulder pain in degenerative disease. In this study, we investigated the clinical outcomes of the BMC injections for treatment of shoulder pain and disability due to osteoarthritis (OA) and rotator cuff tears in a treatment registry population.
Methods: A total of 115 shoulders in 102 patients were treated with autologous BMC injections for symptomatic OA at the glenohumeral joint and/or rotator cuff tears. Data were collected for factors potentially influencing outcome, including age, sex, body mass index, and the type of condition treated (ie, OA or rotator cuff tear). Clinical outcomes were assessed serially over time using the disabilities of the arm, shoulder and hand score (DASH), the numeric pain scale (NPS), and a subjective improvement rating scale. Baseline scores were compared to the most recent outcome scores at the time of the analysis and adjusted for demographic differences. We reported comparisons of pre- and post-treatment scores, the differences between osteoarthritis and rotator cuff groups, and the predictive effects on the clinical outcomes.
Results: At the most current follow-up assessment after treatment, the average DASH score decreased (improved) from 36.1 to 17.1 (P,0.001) and the average numeric pain scale value decreased (improved) from 4.3 to 2.4 (P,0.001). These changes were associated with an average subjective improvement of 48.8%. No differences were observed between outcomes among the shoulders treated for OA versus rotator cuff tears, nor did age, sex, or body mass index influence pain or functional outcomes. There were no significant treatment-related adverse events reported.
Discussion: We observed preliminarily encouraging results following BMC injections for shoulder OA and rotator cuff tears. These results serve as basis for the design of an adequately powered randomized controlled trial
Stem Cell Research and Therapy; Volume 4: Issue 10; 2014, Article ID 370621. Centeno CJ.
Introduction: We investigated the efficacy and safety of autologous bone marrow concentrate (BMC) for the treatment of symptomatic hip osteoarthritis.
Methods: Treatment registry data for 216 hips treated among 196 patients who underwent a BMC procedure for hip osteoarthritis (OA) were analyzed. Data regarding adverse events (AEs), subjective percentage improvement, Oxford Hip Scores (OHS), and numeric pain scale (NPS) scores were assessed and compared to baseline at 1, 3, 6 months, and annually after treatment.
Results: The mean reported subjective percentage improvement across all 216 treated hips was 30.2%. The mean OHS change was 6.4 points improved (p<0.001). The NPS scores from baseline to post treatment decreased from 4.5 to 3.3 (p<0.001). Twelve AEs were reported, none of which were serious or persisting. Patients ≤ 55 years old were substantially more likely to report improvement on the OHS [OR: 11.1 (1.6-77.8)] and also more likely to report greater than 50% improvement on the subjective percentage improvement scale [OR: 2.8 (1.2-6.7)].
Conclusion: The present study of BMC injections for hip OA demonstrated encouraging results for improved outcomes with no significant complications. We found that patients younger than 55 years old were more likely to report improvement on the OHS and subjective percentage improvement scales. Further study with randomized trials is warranted to confirm the reported results.
BioMed Research International; Volume 2014, Article ID 370621. Centeno CJ.
Introduction. We investigated the use of autologous bone marrow concentrate (BMC) with and without an adipose graft, for treatment of knee osteoarthritis (OA). Methods. Treatment registry data for patients who underwent BMC procedures with and without an adipose graft were analyzed. Pre- and posttreatment outcomes of interest included the lower extremity functional scale (LEFS), the numerical pain scale (NPS), and a subjective percentage improvement rating. Multivariate analyses were performed to examine the effects of treatment type adjusting for potential confounding factors. The frequency and type of adverse events (AE) were also examined. Results. 840 procedures were performed, 616 without and 224 with adipose graft. The mean LEFS score increased by 7.9 and 9.8 in the two groups (out of 80), respectively, and the mean NPS score decreased from 4 to 2.6 and from 4.3 to 3 in the two groups, respectively. AE rates were 6% and 8.9% in the two groups, respectively. Although pre- and posttreatment improvements were statistically significant, the differences between the groups were not. Conclusion. BMC injections for knee OA showed encouraging outcomes and a low rate of AEs. Addition of an adipose graft to the BMC did not provide a detectible benefit over BMC alone.
PM & R. 2014 Jan;6(1):70–7. doi: 10.1016/j.pmrj.2013.08.612. Centeno CJ.
Abstract: The use of stem cells in orthopedics has been researched for many years, with robust animal data that show efficacy in cartilage healing, tendon repair, and intervertebral disk treatment. Early clinical data are also just starting to be published, and these results are encouraging. Safety data in large case series, some that lasted for many years, have also been published. The field of tissue engineering with stem cells in musculoskeletal impairments has the potential to reduce morbidity and improve clinical outcomes. The regulatory environment for this area of medicine is still developing.
Copyright © 2014 American Academy of Physical Medicine and Rehabilitation. Published by Elsevier Inc. All rights reserved. PMID: 24439149
Amide-Type Local Anesthetics and Human Mesenchymal Stem Cells: Clinical Implications for Stem Cell Therapy.
In the realm of regenerative medicine, human mesenchymal stem cells (hMSCs) are gaining attention as a cell source for the repair and regeneration of tissues spanning an array of medical disciplines. In orthopedics, hMSCs are often delivered in a site-specific manner at the area of interest and may require the concurrent application of local anesthetics (LAs). To address the implications of using hMSCs in combination with anesthetics for intra-articular applications, we investigated the effect that clinically relevant doses of amide-type LAs have on the viability of bone marrow-derived hMSCs and began to characterize the mechanism of LA-induced hMSC death. In our study, culture-expanded hMSCs from three donors were exposed to the amide-type LAs ropivacaine, lidocaine, bupivacaine, and mepivacaine. To replicate the physiological dilution of LAs once injected into the synovial capsule, each anesthetic was reduced to 12.5%, 25%, and 50% of the stock solution and incubated with each hMSC line for 40 minutes, 120 minutes, 360 minutes, and 24 hours. At each time point, cell viability assays were performed. We found that extended treatment with LAs for 24 hours had a significant impact on both hMSC viability and adhesion. In addition, hMSC treatment with three of the four anesthetics resulted in cell death via apoptosis following brief exposures. Ultimately, we concluded that amide-type LAs induce hMSC apoptosis in a time- and dose-dependent manner that may threaten clinical outcomes, following a similar trend that has been established between these particular anesthetics and articular chondrocytes both in vitro and in vivo.
NOTE: This article was written by an independent third party after requesting the source data for our n=339 safety paper. They reviewed that source data and concluded that it was the highest quality data reporting on safety of any paper they reviewed in the meta-analysis. As a result, it is the primary basis for many of their conclusions below.
Osteoarthritis Cartilage. 2013 Oct;21(10):1465-73. doi: 10.1016/j.joca.2013.06.025. Epub 2013 Jul 4.
Abstract
BACKGROUND: An important goal of stem cell research in orthopaedics is to develop clinically relevant techniques that could be applied to heal cartilage or joint pathology. Stem cell treatment in orthopaedics for joint pathology is promising since these cells have the ability to modulate different processes in the various tissues of the joint simultaneously. The non life-threatening nature of musculoskeletal system disorders makes safety ofstem cell therapy a necessary prerequisite.
OBJECTIVE: To systematically review the literature and provide an overview of reported adverse events (AEs) of intra-articular treatment with culture-expanded stem cells in humans.
DESIGN: A systematic literature search was performed in Pubmed, EMBASE, Web of Science and CINAHL in February 2013. AEs were reported into three categories: local/systemic, serious adverse event or AE (SAE/AE), related/unrelated.
RESULTS: 3039 Potentially eligible articles were identified of which eventually eight fulfilled our inclusion criteria. In total, 844 procedures with a mean follow-up of 21 months were analysed. Autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) were used for cartilage repair and osteoarthritis treatment in all included studies. Four SAEs were reported by the authors. One infection following bone marrow aspiration (BMA) was reported as probably related and resolved with antibiotics. One pulmonary embolism occurred 2 weeks after BMA and was reported as possibly related. Two tumours, both not at the site of injection, were reported as unrelated. Twenty-two other cases of possible procedure-related and seven of possible stem cell-product related adverse events (AEs) were documented. The main AEs related to the procedure were increased pain/swelling and dehydration after BMA. Increased pain and swelling was the only AE reported as related to the stem cell-product.
CONCLUSIONS: Based on current literature review we conclude that application of cultured stem cells in joints appears to be safe. We believe that with continuous caution for potential side effects, it is reasonable to continue with the development of articular stem cell therapies.
Centeno CJ, D Freeman M.
Wien Med Wochenschr. 2013 Aug 15.
ABSTRACT
BACKGROUND: Mesenchymal stem cells (MSCs) show promising clinical potential as multipotent therapeutic agents in regenerative medicine, including a number of orthopedic applications. Objective: To study the possible value of MSC’s injected intra-articular in patients with carpometecarpal (CMC) joint and hand osteoarthritis (OA).
METHODS: This is a prospective, case series with an untreated control that was obtained through a convenience sample. Patients underwent a bone marrow aspiration with isolation and culture expansion of MSC’s using a serum free, autologous platelet lysate. Autologous MSC’s were injected intra-articular utilizing imaging guidance. Percentage improvement, functional and visual analog scale data was collected via survey at pre-procedure, 3 months, 6 months, and annually.
RESULTS: Six OA patients and four controls were recruited. The mean reported pain relief was significantly higher +60% in the thumb OA group (n=6, p=.032) than in the control -18.75% (n=4). The average time reporting was 11.83 +/- 5.70 months and 9.55 +/- 6.49 months for both groups, respectively. On average, a greater than 30% reduction were observed in all VAS scale metrics (n=5), average reporting time was 13 +/-5.52 months. The majority of patients (66.7%, n=6) reported an increase in both strength and range of motion, average reporting time was 11.83 +/- 5.7 months. No complications were reported.
CONCLUSIONS:Percutaneous implantation of cultured MSCs into the carpometecarpal joint was associated with patient reported improvement in pain and function that was not seen in an untreated control. In addition, all patients within this small case series reported no complications.
A Case Series of Percutaneous Treatment of Non-Union Fractures with Autologous, Culture Expanded, Bone Marrow Derived, Mesenchymal Stem Cells and Platelet Lysate
Centeno CJ, Schultz JR, Cheever M, Freeman M, Robinson B, Faulkner S
—Journal of Bioengineering and Biomedical Science 2011.
Abstract
Background: Current treatment options for stable non-union fractures represent major clinical challenges, and are a major health issue. Fracture treatment can take many forms, usually requiring bone grafting and/or revisions of the fracture with open reduction and internal fixation (ORIF). Conservative care options such as bone morphogenic proteins and bone stimulators are also available. The purpose of this study was to determine if culture expanded, autologous MSC’s injected into non-union fractures under c-Arm fluoroscopy could represent an alternative treatment modality in recalcitrant fracture non-unions.
This paper reports on the findings of 6 patients with fracture non-union treated with autologous MSC’s. Patients and methods: We evaluated 6 consecutive patients with chronic fracture non-unions. Patients consisted of 4 women and 2 men with treatment intervention at an average of 8.75 months post-fracture (range 4- 18 months, one patient fracture not included in calculation was >100 mo.).
All treated patients received autologous, culture expanded, mesenchymal stem cells injected percutaneously via fluoroscopic guidance into the site of the fracture non-union. Fracture union was evaluated with the use of follow up high-resolution x-ray and/or CT imaging. Phenotype of the culture-expanded MSCs was evaluated and quantified by flow cytometry of surface antigens.Conclusion: The results of this study support the hypothesis that autologous MSC’s delivered via percutaneous re-implantation may be an alternative modality for the non-operative treatment of recalcitrant non-union fractures.
Safety and Complications Reporting Update on the Re-implantation of Culture-Expanded Mesenchymal Stem Cells using Autologous Platelet Lysate Technique.
Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, Hanson R.–Curr Stem Cell Res Ther. 2011 Oct 17.
Source
The Centeno-Schultz Clinic, Broomfield, Colorado, USA.
Abstract
Mesenchymal stem cells (MSCs) hold great promise as therapeutic agents in regenerative medicine. Numerous animal studies have documented the multipotency of MSCs, showing their capabilities for differentiating into orthopedic tissues such as muscle, bone, cartilage, and tendon. However, the safety of culture expanded MSC’s for human use has only just begun to be reported. Methods: Between 2006 and 2010, two groups of patients were treated for various orthopedic conditions with culture-expanded, autologous, bone marrow-derived MSCs (group 1: n=50; group 2: n=290-one patient in both groups). Cells were cultured in monolayer culture flasks using an autologous platelet lysate technique and re-injected into peripheral joints or into intervertebral discs with use of c-arm fluoroscopy. While both groups had prospective surveillance for complications, Group 1 additionally underwent 3.0T MRI tracking of the re-implant sites. Results: The mean age of patients treated was 53 +/- 13.85 years; 214 were males and 125 females with mean follow-up time from any procedure being 435 days +/- 261 days. Number of contacts initiated based on time from first procedure was 482 at 3 months, 433 at 6 months, 316 contacts at 12 months, 110 contacts at 24 months, and 22 contacts at 36 months. For Group 1, 50 patients underwent 210 MRI surveillance procedures at 3 months, 6 months, 1 year and 2 years which failed to demonstrate any tumor formation at the re-implant sites. Formal disease surveillance for adverse events based on HHS criteria documented significantly less morbidity than is commonly reported for more invasive surgical procedures, all of which were either self-limited or were remedied with therapeutic measures. Two patients were diagnosed with cancer out of 339 patients treated since study inception; however, this was almost certainly unrelated to the MSC therapy and the neoplasm rate in similar to that seen in the U.S. Caucasian population. Knee outcome data was collected on a subset of patients. Here, >75% improvement was reported in 41.4% while decreasing the improvement threshold to >50% improvement, 63.2% reported an improvement. At an average reporting time of 11.3 months from first procedure average reported relief in the knee sample equaled 53.1% (n=133 reporting). Conclusions: Using both intensive high field MRI tracking and complications surveillance in 339 patients, no neoplastic complications were detected at any stem cell re-implantation site. These findings are consistent with our prior publication and other published reports that also show no evidence of malignant transformation in vivo, following implantation of MSCs for orthopedic use.
Osteoblastic differentiation of human and equine adult bone marrow-derived mesenchymal stem cells when BMP-2 or BMP-7 homodimer genetic modification is compared to BMP-2/7 heterodimer genetic modification in the presence and absence of dexamethasone.
Carpenter RS, Goodrich LR, Frisbie DD, Kisiday JD, Carbone B, McIlwraith CW, Centeno CJ, Hidaka C.
Orthopaedic Research Center, Colorado State University, Fort Collins, Colorado 80523.
Abstract
Bone marrow-derived mesenchymal stem cells (BMDMSCs) have been targeted for use in enhancement of bone healing; and their osteogenic potential may be further augmented by genes encoding bone morphogenetic proteins (BMP’s). The purpose of this study was to compare the effect of genetic modification of human and equine BMDMSCs with BMP-2 or -7 or BMP-2 and -7 on their osteoblastogenic differentiation in the presence or absence of dexamethasone. The BMDMSCs were harvested from the iliac crest of three human donors and tuber coxae of three equine donors. Monolayer cells were genetically modified using adenovirus vectors encoding BMP-2, -7 or both and cultured in the presence or absence of dexamethasone. Expression of BMPs was confirmed by enzyme linked immunosorbent assay (ELISA). To evaluate osteoblastic differentiation, cellular morphology was assessed every other day and expression and secretion of alkaline phosphatase (ALP), as well as expression levels of osteonectin (OSTN), osteocalcin (OCN), and runt-related transcription factor-2 (Runx2) were measured for up to 14 days. Human and equine BMDMSCs showed a capacity for osteogenic differentiation regardless of genetic modification or dexamethasone supplementation. Dexamethasone supplementation was more important for osteoblastogenic differentiation of equine BMDMSCs than human BMDMSCs. Genetic modification of BMDMSCs increased ALP secretion with AdBMP-2 homodimer having the greatest effect in both human and equine cells compared to AdBMP 7 or AdBMP 2/7. BMP protein elution rates reached their maximal concentration between day 4 and 8 and remained relatively stable thereafter, suggesting that genetically modified BMDMSCs could be useful for cell-based delivery of BMPs to a site of bone formation. (c) 2010 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res.
Curr Stem Cell Res Ther. 2010 Mar;5(1):81-93.
Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W.
Abstract
ABSTRUCT: Mesenchymal stem cells (MSCs) hold great promise as therapeutic agents in regenerative medicine. Numerous animal studies have documented the multipotency of MSCs, showing their capabilities for differentiating into orthopedic tissues such as muscle, bone, cartilage, and tendon. However, the complication rate for autologous MSC therapy is only now beginning to be reported.
METHODS:
Between 2005 and 2009, two groups of patients were treated for various orthopedic conditions with culture-expanded, autologous, bone marrow-derived MSCs (group 1: n=45; group 2: n=182). Cells were cultured in monolayer culture flasks using an autologous platelet lysate technique and re-injected into peripheral joints (n=213) or into intervertebral discs (n=13) with use of c-arm fluoroscopy. While both groups had prospective surveillance for complications, Group 1 additionally underwent 3.0T MRI tracking of the re-implant sites.
RESULTS: Mean follow-up from the time of the re-implant procedure was 10.6 +/- 7.3 months. Serial MRI’s at 3 months, 6 months, 1 year and 2 years failed to demonstrate any tumor formation at the re-implant sites. Formal disease surveillance for adverse events based on HHS criteria documented 7 cases of probable procedure-related complications (thought to be associated with the re-implant procedure itself) and three cases of possible stem cell complications, all of which were either self-limited or were remedied with simple therapeutic measures. One patient was diagnosed with cancer; however, this was almost certainly unrelated to the MSC therapy.
CONCLUSIONS: Using both high field MRI tracking and general surveillance in 227 patients, no neoplastic complications were detected at any stem cell re-implantation site. These findings are consistent with other reports that also show no evidence of malignant transformation in vivo, following implantation of MSCs that were expanded in vitro for limited periods. PMID: 19951252
Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D.
Regenerative Sciences Inc, Centeno-Schultz Clinic, Westminster, CO 80020, USA.
–Med Hypotheses. 2008 Dec;71(6):900-8. Epub 2008 Sep 10.
Abstract
Mesenchymal stem cells are pluripotent cells found in multiple human tissues including bone marrow, synovial tissues, and adipose tissues. They have been shown to differentiate into bone, cartilage, muscle, and adipose tissue and represent a possible promising new therapy in regenerative medicine. Because of their multi-potent capabilities, mesenchymal stem cell (MSC) lineages have been used successfully in animal models to regenerate articular cartilage and in human models to regenerate bone. The regeneration of articular cartilage via percutaneous introduction of mesenchymal stem cells (MSC’s) is a topic of significant scientific and therapeutic interest. Current treatment for cartilage damage in osteoarthritis focuses on surgical interventions such as arthroscopic debridement, microfracture, and cartilage grafting/transplant. These procedures have proven to be less effective than hoped, are invasive, and often entail a prolonged recovery time. We hypothesize that autologous mesenchymal stem cells can be harvested from the iliac crest, expanded using the patient’s own growth factors from platelet lysate, then successfully implanted to increase cartilage volume in an adult human knee. We present a review highlighting the developments in cellular and regenerative medicine in the arena mesenchymal stem cell therapy, as well as a case of successful harvest, expansion, and transplant of autologous mesenchymal stem cells into an adult human knee that resulted in an increase in meniscal cartilage volume.
Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D.
Regenerative Sciences Inc (RSI), Centeno-Schultz Clinic, Westminster, CO 80020, USA.–Pain Physician. 2008 May-Jun;11(3):343-53.
Abstract
BACKGROUND: The ability to repair tissue via percutaneous means may allow interventional pain physicians to manage a wide variety of diseases including peripheral joint injuries and osteoarthritis. This review will highlight the developments in cellular medicine that may soon permit interventional pain management physicians to treat a much wider variety of clinical conditions and highlight an interventional case study using these technologies OBJECTIVE: To determine if isolated and expanded human autologous mesenchymal stem cells could effectively regenerate cartilage and meniscal tissue when percutaneously injected into knees. DESIGN: Case Study SETTING: Private Interventional Pain Management practice. METHODS: An IRB approved study with a consenting volunteer in which mesenchymal stem cells were isolated and cultured ex-vivo from bone marrow aspiration of the iliac crest. The mesenchymal stem cells were then percutaneously injected into the subject’s knee with MRI proven degenerative joint disease. Pre- and post-treatment subjective visual analog pain scores, physical therapy assessments, and MRIs measured clinical and radiographic changes. RESULTS: At 24 weeks post-injection, the patient had statistically significant cartilage and meniscus growth on MRI, as well as increased range of motion and decreased modified VAS pain scores. CONCLUSION: The described process of autologous mesenchymal stem cell culture and percutaneous injection into a knee with symptomatic and radiographic degenerative joint disease resulted in significant cartilage growth, decreased pain and increased joint mobility in this patient. This has significant future implications for minimally invasive treatment of osteoarthritis and meniscal injury.
PMID: 18523506 [PubMed – indexed for MEDLINE]Free Article
Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: A case study.
Centeno CJ, Kisiday J, Freeman M, Schultz JR.
The Centeno-Schultz Clinic, 11080 Circle Point Road, Building 2, Suite 140, Westminster, CO 80020, USA. centenooffice@cenenoclinic.com
–Pain Physician. 2006 Jul;9(3):253-6.
Abstract
HISTORY: This is a case report of a 64-year-old white male with a 20 year history of unilateral hip pain that had become debilitating over the last several years. On intake, Harris hip score was rated as: Pain subscale = 10, Function subscale = 32, Deformity subscale = 4, Motions subscale = 4.775 with a total score of 50.8 out of 100. MRI of the affected hip showed severe degeneration with spurring, decrease in joint space, and several large subchondral cysts. The patient had been evaluated by an orthopedic surgeon and told he was a candidate for bipolar hip replacement. METHOD: Two autologous nucleated cell collections were performed from bone marrow with subsequent isolation and transfers into the intra-articular hip using a hyaluronic acid and thrombin activated platelet rich plasma scaffold. Marrow samples were processed by centrifugation and lysis techniques to isolate nucleated cells. CONCLUSION: This report describes partial by articular surface regeneration 8 weeks after intraarticular bone marrow transfer. Post-op 3.0T FGRE MRI showed neocortex formation when compared to immediate pre-op MRI and objective improvements were noted that coincided with subjective reports of improvement.
PMID: 16886034 [PubMed – indexed for MEDLINE]Free Article
In fact, as of August of 2015, the n of all Regenexx medical group patients published in the world medical-research literature in bone marrow stem cells used to treat orthopedic conditions constituted 33% of the total n published in the U.S. National Library of Medicine on that topic. In other words, as of that date, there is no other author in the United States or worldwide that has published more clinical data on these procedures in more common indications such as knee OA, shoulder OA and rotator cuff tear, ankle OA, or knee ACL tears. In addition, our most recently accepted work that will soon be published in a high-impact-factor orthopedics journal will be a safety paper that followed 2,372 patients for up to nine years and that used independent, blinded adjudication of SAEs. Once it’s published, it will be the largest complications tracking paper of its kind for any indication in stem cells worldwide.
As a comparison, we know of no other U.S. medical practice that has tracked the outcomes and complications of its patients since the inception of BMC or expanded MSC care or published that data in the massive numbers that our practice has made available. So for clinical research, we have been and remain the world leaders in publishing registry data.
Ongoing Clinical and Lab Research
On the lab-research front, we continue to innovate. For example, our group medical practice is using its resources to investigate the microenvironment (ME) of knee OA patients. We are using multiplex microarray ELISA to measure 25 growth factors and cytokines in patients undergoing our BMC procedure. The goal is to tie this ME to outcome (i.e., do patients with a toxic and proinflammatory ME have a poorer outcome than other patients who have a better ME?). The second is a long-term investigation to determine if controlling the ME in select patients will allow us to improve outcome. We know of no other medical practice in the U.S., or worldwide, that is working at this sophistication level trying to optimize BMC therapies for knee OA.
Hence, in support of the claims for orthopedic BMC-treated patients, as far as research we have the following:
- The world’s largest registry tracking patient outcomes and complications
- The most research based on patient n published out of this registry
- Ongoing research projects that are more sophisticated than other group’s using BMC
Conclusions
The Regenexx medical group doesn’t make claims lightly. Unlike a medical practice that buys an automated bedside centrifuge and takes a weekend course on how to perform a BMA and use BMC or PRP, as the evidence shows, we are quite different. Everything from the back-end lab research to the way BMA is acquired, processed, dosed, and reinjected is more advanced than what is being commonly performed by other physicians.
Can a stem cell procedure help me? To find out if you might be a candidate for a Regenexx stem cell procedure, complete our Regenexx Procedure Candidate Form online.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.