2023 PRP RCT Infographic and Study Failure Analysis
Almost every year, I try to spend significant time diving into the literature on interventional orthobiologics and summarizing it. This helps me understand where we are in the evolution of the literature and hopefully provides some insight into what’s working and what’s not. This year I summarized the entire published literature on PRP injections for all musculoskeletal indications, and I wasn’t disappointed. In addition, this year’s review was done with an eye toward explaining why a few papers show PRP failing as a treatment that don’t agree with the rest of the published literature. Let’s dig in.
My Prior PRP Reviews
I’ve done a few prior literature reviews on PRP, listed here:
- PRP RCTs 2019
- Knee Arthritis PRP RCTs 2020
- PRP Knee Arthritis RCTs 2022
- PRP RCTs for Carpal Tunnel 2022
- PRP RCTs in Spine 2023
My 2023 PRP Review
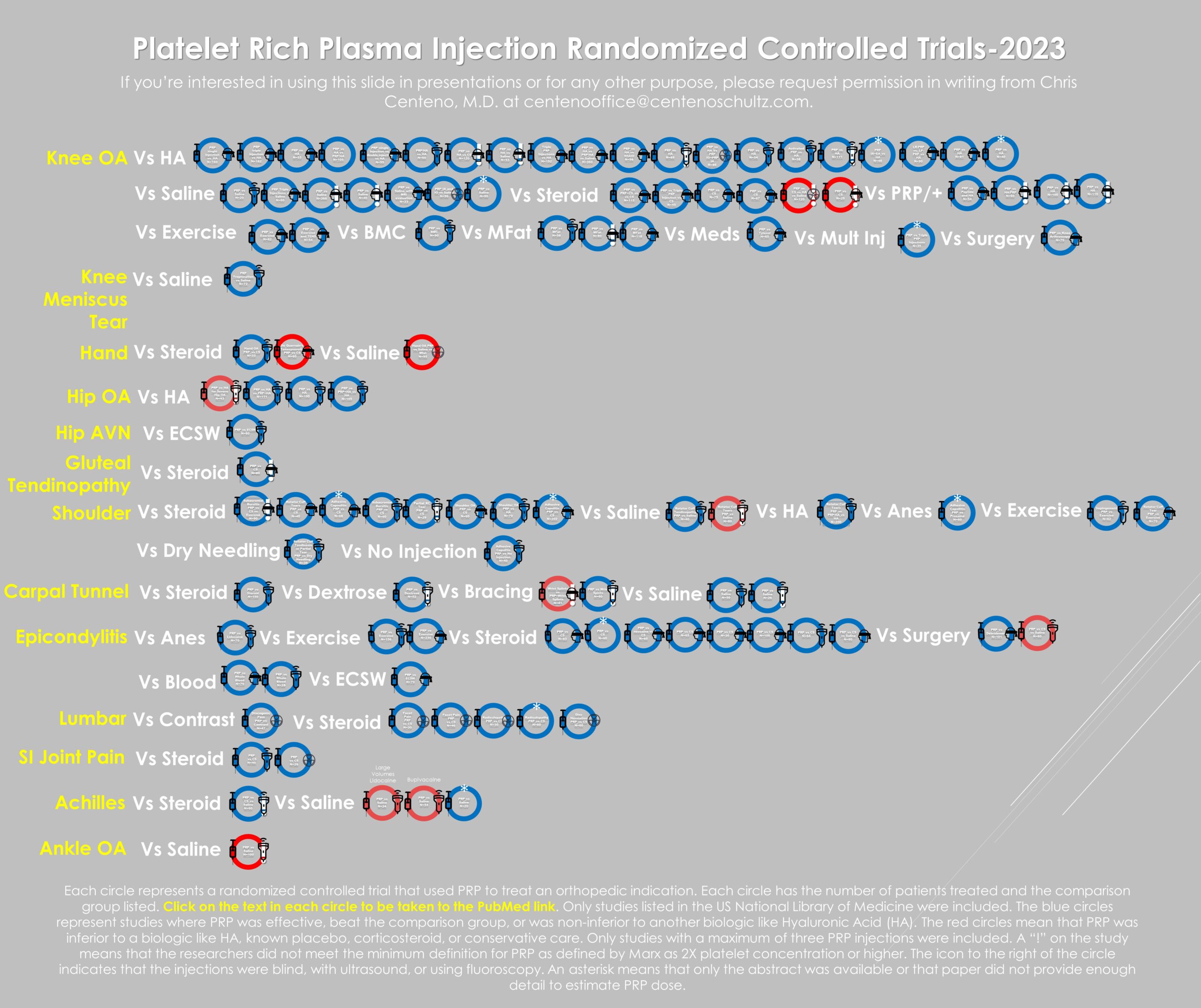
Above, you see the infographic I created, which now includes 107 Randomized Controlled Trials (RCTs) that have tested PRP against many different placebos and traditional treatments. My first thought was, “Wow”! My second thought after pulling this together was that we now have better literature support for the wide use of PRP in many orthopedic indications than we have for orthopedic surgery as a field. How do I know that? Because in 2021, a systematic review was published on ten common orthopedic surgeries that showed that only one had high-level research support.
For this review, I downloaded or purchased all of these papers. That was a big job that took about 20 hours, during which I had the privilege of “renting” a dozen or more of the papers for far too much money. However, the best thing since sliced bread was my DeepDyve subscription which allowed me to read about half of these without paying a per-click fee.
The infographic above represents each study with a circle, each with a link to PubMed. There are a couple of icons used. First, if the study circle has an asterisk, I could not get the full text, or the paper never discussed how they made the PRP. The icons on the right indicate how the injection was done. The blindfold means that the injection was blind, the ultrasound probe means that the injection was guided using that technology, and the radiation icon means fluoroscopy guided. Finally, an exclamation point means the study couldn’t meet the two times platelet concentration standard. Sometimes you’ll also see my notes.
Analysis of Studies That Show a Failure of PRP Injections
If we define a PRP study failure as that substance being less effective than hyaluronic acid or not beating either a steroid shot or saline, then 11 studies meet that definition of failed, which is 10% of the total studies. The red circles represent these outcome failures. Conversely, 90% showed success (blue circles). Why would a PRP study failure happen?
I’ve blogged before that in some of the studies where PRP fails to beat placebo; the research didn’t actually use PRP as defined by Marx (at least two times the concentration of platelets over what’s present in whole blood). That’s a problem because the experimental data generated by our team would suggest that for older patients, this concentration is critical (1). It’s also an issue because these papers represented that they were using PRP but never met the widely accepted definition, so there’s a bit of unintentional (or intentional?) deception involved.
To identify the papers that used below-threshold platelet treatments, I read every one of these studies and looked for the telltale signs of not meeting this minimum concentration level. How was that done?
First, I paid attention to the volume of whole blood drawn and the volume of PRP obtained. A great example of this problem is represented by the two most commonly used kits in Europe-Arthrex ACP and RegenLab. Both begin with an 8-10 ml blood draw and commonly output 3-4 ml of product to inject. If we use an 8ml draw that outputs 4 ml of “PRP,” then that could be 2X concentration if the efficiency of platelet isolation was 100%. However, based on independent lab data, the Arthrex machine is only 48% efficient, and the RegenLab kit is only 46%. So that means in a real-world test, Arthrex-ACP produces a 1.3X, and RegenLab produces a 1.6X PRP. Hence papers that used these kits were labeled as not being about PRP based on the existing data and flagged with an exclamation point.
Second, the less common problem is one of platelet dose. Only one of these eleven failure papers fell into his category, which was a carpal tunnel “PRP” paper that only injected 0.8-1 ml of PRP made from a 10 ml blood draw. As an expert in interventional orthobiologics who has treated more carpal tunnel cases than I can count, the average injection volume would be 3-5 ml. The paper’s author claimed a 5X concentration, which seemed reasonable because of the double spin isolation technique described. However, they shorted the patient’s total platelet dose by only injecting 1 ml.
If we look at below platelet threshold or “fake PRP” study failures, 6/10 or 60% of total outcomes failures were in this category. In contrast, only 5/101 papers, or 5%, that used real PRP were in this outcome failure category. Hence, by using less than the threshold dose PRP, you raise the risk of study failure by twelve times.
If we look at the same dose threshold in papers that had reported success, then 15/101 or approximately 15% of the successes used this form of injection. Why would this type of inadequate platelet dose injectate work at all? More on that below.
Why Would Below-Threshold PRP Injections Work?
Our research team began studying how the dose of platelet growth factors impacts stem and other cells quite by accident way back in 2005. This happened because we were using the patient’s own PRP to make platelet lysate and using that to help culture expand their bone marrow mesenchymal stem cells (MSC). What we noted was that in young patients, we could use 1X of these growth factors, and their cells would grow well in culture. However, for older patients, higher concentrations were needed to maximize MSC growth. We also later published this phenomenon when we did the same thing using young versus old tendon cells (tenocytes) (1).
Hence, if you have a younger patient population in your study, you can probably get away with an injection below 2X platelets. If you have older patients, this becomes a bigger problem.
Why Would There Be Any Failures Then in Studies That Used Real PRP?
One of the things I indexed above was if the injection was blind or not. Why? An example is a knee intra-articular injection which, when performed without imaging guidance, is not in the joint about 20-25% of the time. We also know that blind injections are associated with worse outcomes (2-4). Hence, these blind studies are more technique and operator dependent, with some of the failures likely being due to a provider who wasn’t hitting the tissue target.
Second, what else is injected is also critical. For example, we know that some of these failed studies also injected cell toxic anesthetics like Marcaine (bupivacaine) and Lidocaine (5).
Third, we know that prior corticosteroid injections in a specific area can lead to higher surgical failure rates (6-8). Why? These toxic drugs reduce the ability of an area to heal (9-12). Almost none of these studies report when or if patients had a prior toxic steroid injection.
Using This Infographic
You are free to use this infographic in your talks if you give me credit, and the purpose is to educate physicians. You can not use it in your personal practice marketing nor use it to market your practice or company.
The upshot? I am blown away by the number of published RCTs that exist for PRP and how many get added each year. While we have a “fake PRP” problem that raises the risk of study failure by 1,200%, as long as we know which studies these are, we can discuss these in context. However, with a 90% RCT success rate and >100 successful outcomes, the available research is now BETTER than the literature base that supports the entire field of orthopedic surgery.
__________________________________________
References:
(1) Berger DR, Centeno CJ, Steinmetz NJ. Platelet lysates from aged donors promote human tenocyte proliferation and migration in a concentration-dependent manner. Bone Joint Res. 2019 Feb 2;8(1):32-40. doi: 10.1302/2046-3758.81.BJR-2018-0164.R1. PMID: 30800297; PMCID: PMC6359887.
(2) Bum Park Y, Ah Choi W, Kim YK, Chul Lee S, Hae Lee J. Accuracy of blind versus ultrasound-guided suprapatellar bursal injection. J Clin Ultrasound. 2012 Jan;40(1):20-5. doi: 10.1002/jcu.20890. Epub 2011 Oct 28. PMID: 22033897.
(3) Fang WH, Chen XT, Vangsness CT Jr. Ultrasound-Guided Knee Injections Are More Accurate Than Blind Injections: A Systematic Review of Randomized Controlled Trials. Arthrosc Sports Med Rehabil. 2021;3(4):e1177-e1187. Published 2021 Jun 26. doi:10.1016/j.asmr.2021.01.028
(4) Lundstrom ZT, Sytsma TT, Greenlund LS. Rethinking Viscosupplementation: Ultrasound- Versus Landmark-Guided Injection for Knee Osteoarthritis. J Ultrasound Med. 2020 Jan;39(1):113-117. doi: 10.1002/jum.15081. Epub 2019 Jun 25. PMID: 31237389.
(5) Dregalla RC, Lyons NF, Reischling PD, Centeno CJ. Amide-type local anesthetics and human mesenchymal stem cells: clinical implications for stem cell therapy. Stem Cells Transl Med. 2014 Mar;3(3):365-74. doi: 10.5966/sctm.2013-0058. Epub 2014 Jan 16. PMID: 24436443; PMCID: PMC3952925.
(6) Wijn SRW, Rovers MM, van Tienen TG, Hannink G. Intra-articular corticosteroid injections increase the risk of requiring knee arthroplasty. Bone Joint J. 2020 May;102-B(5):586-592. doi: 10.1302/0301-620X.
(7) Richardson SS, Schairer WW, Sculco TP, Sculco PK. Comparison of Infection Risk with Corticosteroid or Hyaluronic Acid Injection Prior to Total Knee Arthroplasty. J Bone Joint Surg Am. 2019 Jan 16;101(2):112-118. doi: 10.2106/JBJS.18.00454.
(8) Ravi B, Escott BG, Wasserstein D, Croxford R, Hollands S, Paterson JM, Kreder HJ, Hawker GA. Intraarticular hip injection and early revision surgery following total hip arthroplasty: a retrospective cohort study. Arthritis Rheumatol. 2015 Jan;67(1):162-8. doi: 10.1002/art.38886.
(9) McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA.2017;317(19):1967–1975. doi: 10.1001/jama.2017.5283
(10) STEINBROCKER O, EHRLICH ME, SILVER M, SICHER W, BERKOWITZ S, CARP S, FEISTEIN H. The clinical application of cortisone and ACTH in arthritis and related conditions: methods and problems. II: Side effects complications, contraindications, precautions and conclusions. Ariz Med. 1951 Sep;8(9):29-35.
(11) Wang BL, Sun W, Shi ZC, et al. Decreased proliferation of mesenchymal stem cells in corticosteroid-induced osteonecrosis of femoral head. Orthopedics. 2008;31(5):444. doi:10.3928/01477447-20080501-33
(12) Bonnevialle N, Bayle X, Projetti F, Wargny M, Gomez-Brouchet A, Mansat P. Variations of the micro-vascularization of the greater tuberosity in patients with rotator cuff tears. Int Orthop. 2015;39(2):371‐376. doi:10.1007/s00264-014-2628-z

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.