Why Can’t We Be Friends? A Regenative Labs Update

Credit: Shutterstock
It seems like such a simple missive, “Why can’t we be friends?” Who could possibly disagree with that concept? This is what a company called “Regenative Labs” has been pinging me with on LinkedIn. What’s that all about and how could I possibly refuse? Let’s dig in.
Regenative Pings Me
Several weeks ago, I started to get pinged on LinkedIn by Regenative Labs, a birth tissues company. I largely ignored these, partly because I have blogged on these guys before and because I had more pressing things to handle. One of those pings was about a recent legal opinion sourced by the company and then the second massive salvo had to with a call I made. More on those issues below.
A Cell Phone Crash
Yesterday, when returning from visiting my mother in Florida, my phone locked me out. The new model I have has been known to have some early bugs, so the suggestions online were to reboot the phone for a factory reset. That’s painful as it means you need to reinstall all of your apps manually one by one and then log into each one. Several hours into that process, I finally got LinkedIn back up and running and for some reason, the app decided to show me all of the pings I had from posts by Regenative Labs. I literally couldn’t find the notifications I was interested in because of page after page of Regenative pings. Hence, after ignoring these guys for weeks, I thought it was time for my response.
Why Can’t We Be Friends?
I love that 70s song and what’s not to like about the concept. In this crazy COVID world and divisive political environment we’ve created, it would seem like a great social media meme. What jerk could refuse such an innocent request?
This is the theme that a Regenative Labs company executive used several weeks ago that began to blow up my notification panel. However, in my opinion, in context, as he used it, to me the phrase really means something like, “Why can’t we do business?” So let’s dive into that today.
My “Friends” Criteria
I chose our partners for Regenexx very carefully. They have to meet several different rigorous criteria. So let’s compare Regenative Labs as a company to my “friends” criteria for our business partners. I want our “friends” to be:
- Regulatory Compliant
- Only make claims that can be backed up-meaning less hype and more substance
- Research-based in that clinical studies show that the product can be helpful to my patients
- Forthright-meaning no games
So how does Regenative Labs match up? Let’s start at the beginning.
What Is Regenative Labs?
First, note that the name of this company is “Regenative” without the “er” found in the word “Regenerative”. Based on my experience with the company, Regenative is primarily a sales and marketing company that processes birth tissues. They manufacture several birth tissue products including one that is made from Wharton’s Jelly.
Regulatory Compliant
What I mean here is that the company has a history of being compliant with FDA, FTC, and these days Medicare/CMS regulations. So let’s explore this with Regenative.
I’ve blogged before about Regenative Labs, as it first came on my radar in 2019. That was around the time of the 2019 A4M conference in Las Vegas. After giving my lecture, I strolled through the vendor hall and found many booths making claims that seemed to me to be inconsistent with a recent FDA notice. This is what the Regenative booth proclaimed at that time:

What were the issues? Check out these blowups of the above snaps:
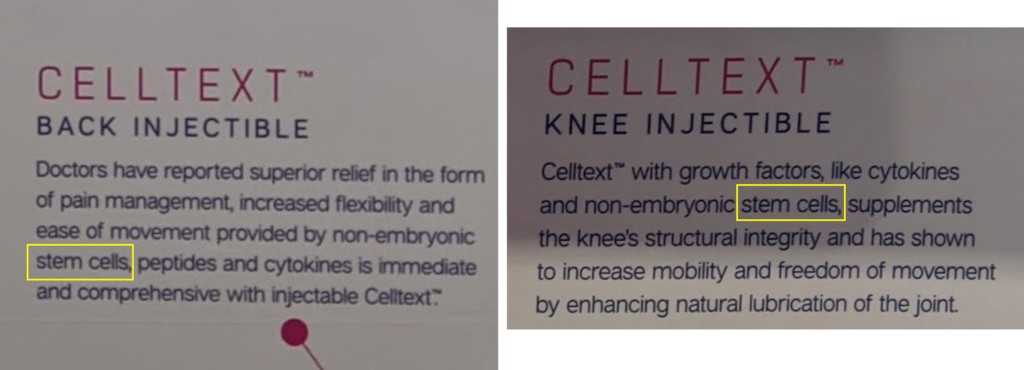
Back then it was very clear, based on a slew of FDA Warning and Untitled Letters, that claiming to sell stem cells in a vial was not consistent with FDA regulations. Hence, IMHO that’s a clear loss of Centeno “friend points”.
Then Regenative jumped in with both feet on the Medicare reimbursement game, claiming that Medicare covered its products for many common orthopedic conditions (this is a sales brochure sent to me by a colleague):

The problem here was this Q and A by Peter Marks (head of the FDA CBER division) where he stated that Medicare Q-codes like the one Regenative claimed to have were issued in error with FDA asleep at the switch. Then this warning from a healthcare law firm discussing how its clients who were billing Medicare for birth tissues for orthopedic use were getting letters from Medicare which could lead to painful clawbacks. Another big friend points deduction.
Frankly, as of the middle of last year, I was sure companies like Regenative were dead in the water. Add to that the expiration of the FDA enforcement discretion period where many such birth tissue vendors folded their tents and I was surprised to get emails from colleagues recently that Regenative was still around and kicking.
However, then the company recently posted a legal opinion letter by none other than Christine Humphreys, who was one of my attorneys back in the day. The opinion seemed to state that what Regenative was making was “minimally manipulated” and solely regulated as a tissue and not as a drug as FDA has so famously claimed with other similar products. Regrettably, after reading the letter and asking a legal expert I trust, neither of us believes that the FDA will back off based on the opinion. However, time will tell on that one.
So in the end, did Regenative pass my “Regulatory” friend test? Nope.
Only Make Claims that Can Be Backed Up
The next friend test is simple, less hype, and more substance. So how does Regenative stack up?
The “stem cell” claim for birth tissues piqued my interest back in about 2014. At the time there were a handful of companies claiming to sell amniotic tissue that had living and functional mesenchymal stem cells. We tested that claim in our lab and the product we tested had no living cells, let alone stem cells. Then we tested a few more products and got the same result. Ultimately other labs tested the same stuff and found the same thing (1-4). Finally, many of the companies switched from amniotic to umbilical cord products, and the claims of live and functional stem cells continued, thus so did our testing. Eventually, we tested those umbilical products as did Lisa Fortier of Cornell and neither of us found any live and functional mesenchymal stem cells. So while Regenative wasn’t the only company at the time making this claim, they don’t earn any Centeno “friend points” by making it.
Research-Based
Is there any meaningful clinical research showing that Regenative’s umbilical cord or other products can help my patients with problems like knee arthritis, low back pain, or shoulder rotator cuff tears? I am aware of no credible clinical research that has been conducted and published using these products.
No Games
This last part is what I call the “X” factor. Meaning that this is where points can be earned or lost based on stuff that’s intangible. Certainly, Regenative pinging me on Linkedin using the lyrics from a 70’s song is not a net positive. Or at least the way the company tried to go about it.
On the topic of the phone call mentioned above, how did that happen? As part of my research on another blog, I had looked up the corporate registration for Regenative:
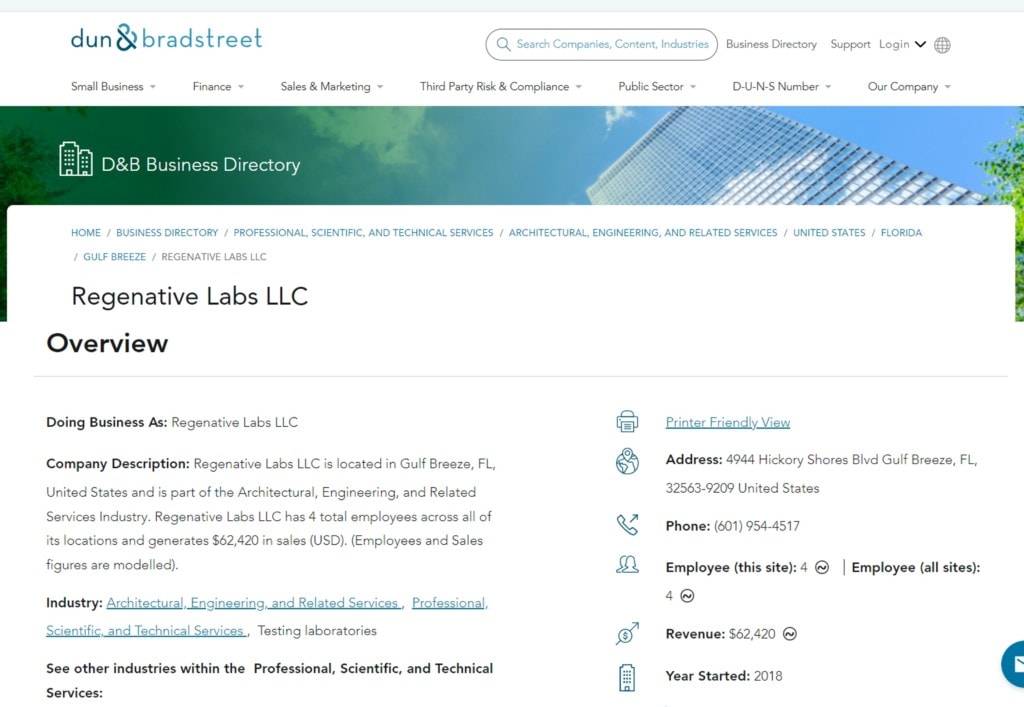
Note the official address on the registration is 4944 Hickory Shores; Gulf Breeze, FL. I ran a quick Google search on that address, expecting to find an office building, but here’s what I found:
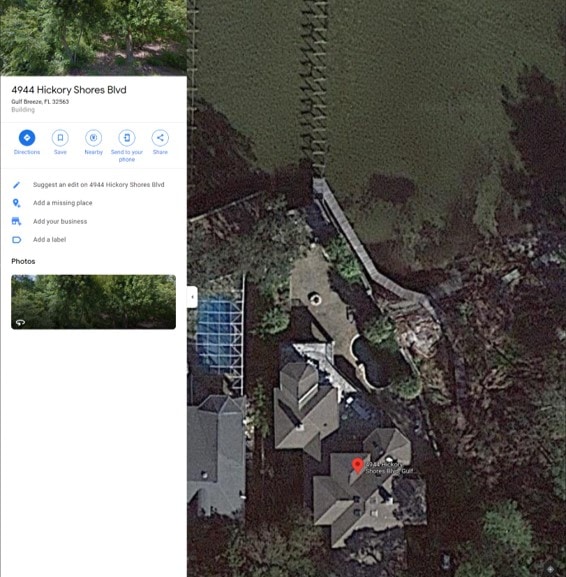
That’s a home owned by Mitchell “Chad” Barrett:
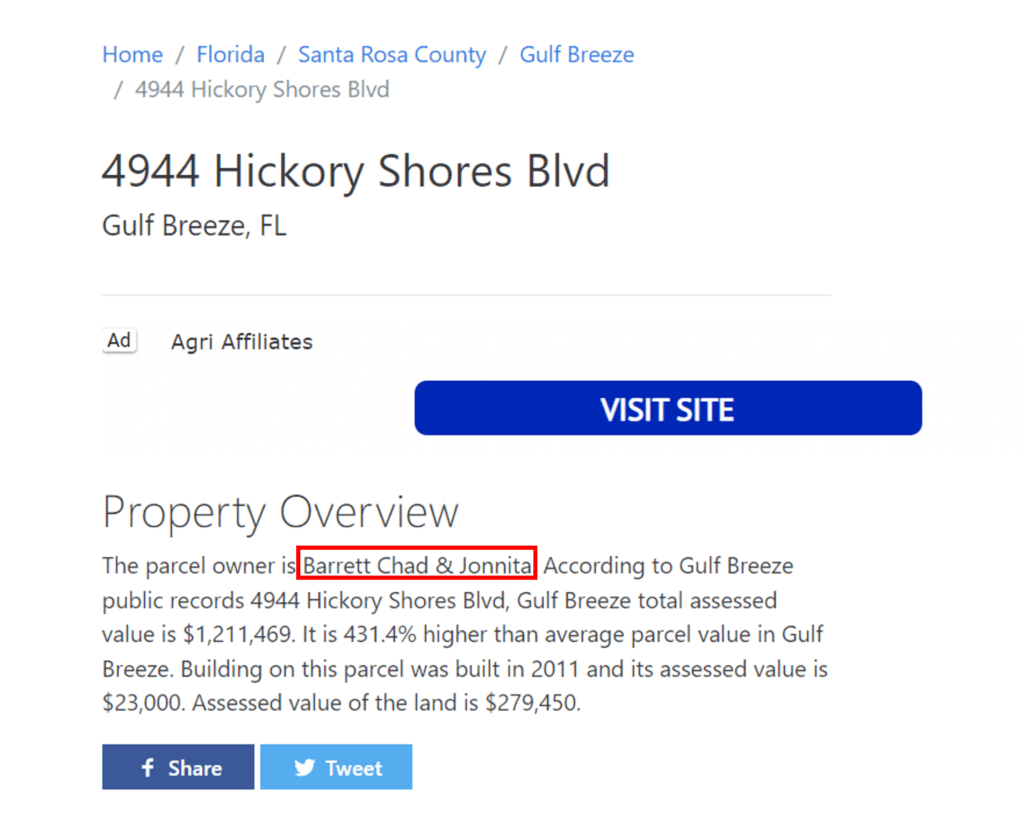
Hence, I decided to call the number listed, as this didn’t make much sense to me.
A Chance Phone Call
As anyone calling a company would expect, I thought that I would get the company’s front desk, but instead, I got the personal line of Tyler Barrett (son of Mitchell “Chad” Barrett) and CEO of Regenative.
Tyler and I spoke for about 10 minutes and here’s what I recall from that conversation:
- Tyler stated that he is no longer close to his dad.
- Tyler claimed that his father is not involved with Regenative in any way.
- When I relayed some of the information I was exploring for the blog, Tyler abruptly hung up.
Also during our talk, he admitted:
- That the products his company sells have no mesenchymal stem cells. He stated that he felt his website was now FDA compliant.
- That he would fire any sales rep claiming that his products could be billed to Medicare for something like an injection to treat knee arthritis. That was a reference to the flyer I showed above, which he claimed had been sent by a rouge company salesperson who was fired shortly thereafter.
Summary
So how did Regenative do on the Centeno “friend score”?
- Regulatory-points deducted
- Claims-points deducted
- Research-points deducted
- X-factor (Games)-Company address listed at Dad’s house, not sure what to make of that…
So do I want to work with Regenative Labs? Not based on my personal assessment.
The upshot? Why can’t we be friends sounds so innocent until you break down what that actually means. Based on my personal rating system, I think I’ll stay out of the friend zone for now.
______________________________________
References:
(1) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Oct;49(12):3404-3413. doi: 10.1177/03635465211031275. Epub 2021 Aug 16. PMID: 34398643.
(2) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(3) Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. Am J Sports Med. 2019 Apr;47(5):1230-1235. doi: 10.1177/0363546519829034. Epub 2019 Mar 7. PMID: 30844295.
(4) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
