The RegenLabs Saga Continues…
Frankly, at this point, my favorite saying, “You just can’t make this stuff up” keeps proving true so often, that even I’m astounded. This morning we’ll delve deeper into a company that I thought frankly would be long since gone by now, but continues to impress me with what I consider hijinks and insanity. So let’s dive in on that subject to get updated on what’s up with Regenative Labs.
The Regenative Labs Saga
Regenative Labs is a birth tissue vendor. They came on my radar some time ago for claiming that they were selling a mesenchymal stem cell umbilical cord product. Based on our joint peer-reviewed and published research with CSU on similar products, in my expert opinion, that’s not a credible claim. They then again popped up as one of several companies that had been granted a Q-code for product reimbursement by CMS while the FDA was asleep at the switch (based on Peter Marks’ statements). The company then claimed that you could get paid by Medicare to use its product to treat various orthopedic problems. Shortly thereafter a law firm put out a warning that physicians who were billing birth tissues in this manner were getting Civil Investigative Demands (CIDs). That’s the beginning of a possible clawback where providers repay big bucks back to Medicare, so not good. Then Medicare put out a system-wide memo to shut down all such birth tissue billing practices and begin asking for what was likely hundreds of millions in repayments by doctors who had billed these products to treat problems like arthritis, spinal pain, and tendinopathy. It all seemed gloomy for RegenLabs at that point, but to everyone’s surprise, they sued CMS. Hence, CMS put out another memo that rather than denying every claim like this all at once, they would review each one. The implication from my contacts in the know was that CMS would just deny these claims one by one, basically neutralizing the Regenative Labs suit before it could get going.

Credit: Shutterstock
You Can’t Make This Stuff Up-Hiding in Plain Sight
IMHO, if you’re Regenative Labs you now have a massive problem. You have a Q-code, but you can bet that every Medicare region has flagged that code for manual review. That means people who work for Medicare will closely scrutinize every claim. However, there’s also an opportunity if you think about this creatively. Medicare, like everyone else, has to educate reviewers about what to look for to deny these claims. These reviewers also look at thousands of other claims a year each, so if you can hide in plain sight, you can confuse those reviewers so that claims go through despite the scrutiny. What does that mean? Let me explain.
I was recently sent information about a Regenative Labs seminar by a colleague. It’s basically a documentation course given by a physician by the name of Scott M. Martin, M.D. Here’s a slide from that presentation:

What’s here? Rather than dictating this procedure as a regenerative medicine injection for a shoulder rotator cuff tear, this documentation is much closer to a surgical procedure. The Wharton’s Jelly injection becomes a “flowable structural tissue allograft transplant”. In my opinion, from reviewing many slides presented, they all take a regenerative medicine injection and try to paint it as a surgical procedure that’s just using another one of many different structural allograft tissues.
Why do this? IMHO because you can hide in plain sight. The Medicare reviewer is one of the thousands of Medicare contractor employees distributed among a dozen different regions who may not fully understand what it is they’re supposed to be paying attention to for this flagged Q-code. In fact, keeping these employees up to date on what to review, approve, and deny is likely a Herculean task. Hence, if you dress up this injection to look like every other surgical structural allograft coming through the system, chances are, more often than not, it will get approved for payment. When it gets denied, you just resubmit it, hoping to get a reviewer who didn’t quite get the memo.
While this solves a short-term problem, it also creates a long-term one. You see, there’s nothing at all permanent about a Medicare approval for payment. Medicare can claw back that money at any time. In addition, if it’s found that you were doing things to try to jigger the system so that it pays when it shouldn’t, then that’s Medicare fraud with 10 years in federal prison for each bill. So is giving a billing course with the exact language that seems to be getting these claims paid just providing valuable information to providers or is it teaching them how to commit Medicare fraud?
Who is Scott M. Martin, M.D.?
I’ve reviewed enough of these birth tissue companies to always take a close look at their medical directors. Why? Because most of these companies, in my opinion, live on the edge of credibility, hence when a physician decides to work for them, there’s usually a reason. I was surely not disappointed when I looked up Scott M Martin, M.D.
This is Dr. Martin’s Linkedin page where he states that he is the VP of Clinical Development for Regenative Labs:
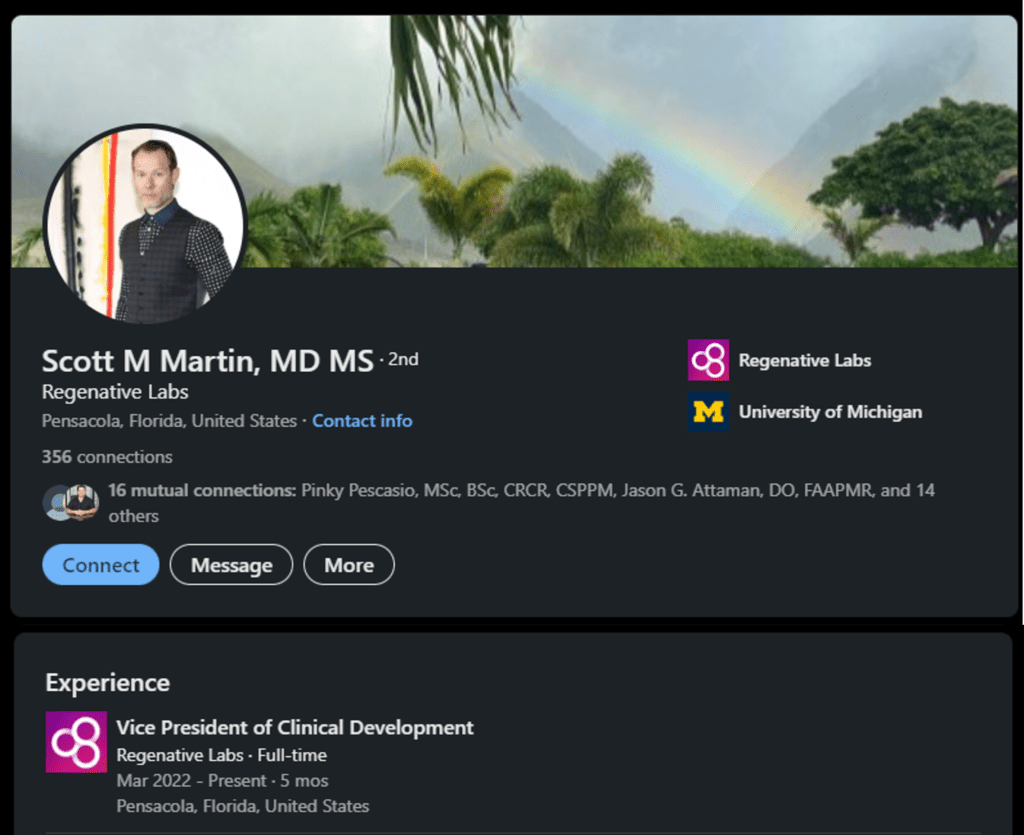
Turns out that Scott was charged by the Nevada Medical Board for:

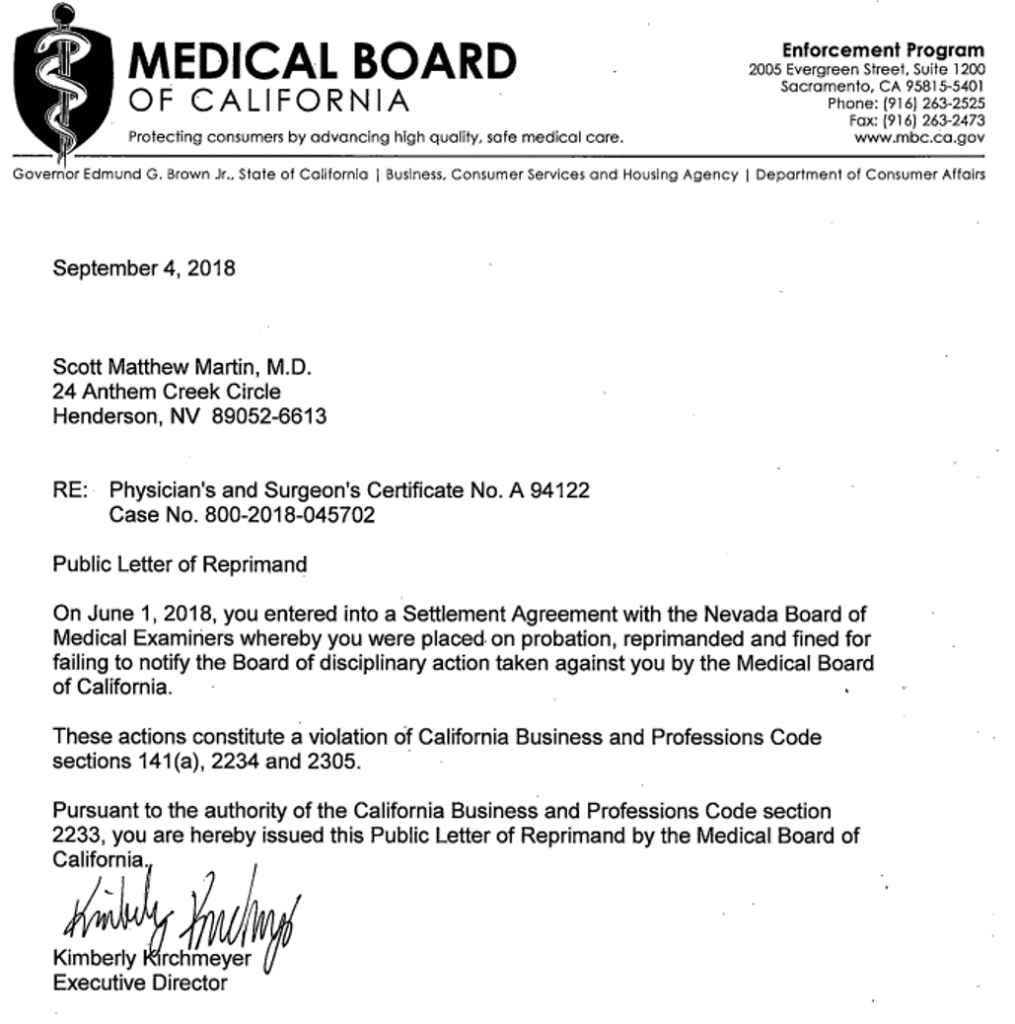
Basically, Scott M Martin, M.D. had his medical license revoked in California and then reinstated with 3-years probation and then failed to inform the Nevada licensing board about these problems. This California license issue was for:
- Gross Negligence
- Repeated Negligent Acts
- Prescribing without an exam or indiction
- Excessive prescribing
- Inadequate records
- Prescribing to an addict
Dr. Martin was fined and placed on probation by the Nevada board. The California board then reprimanded him in Sept 2018 for not informing them about the Nevada board’s action:
A Bigger Problem
In my opinion, Regenative now has a bigger problem than Medicare and its contractors flagging their Q-code for records review. That’s the FDA. Note that this is what’s on the Regenative Labs website as of this morning:
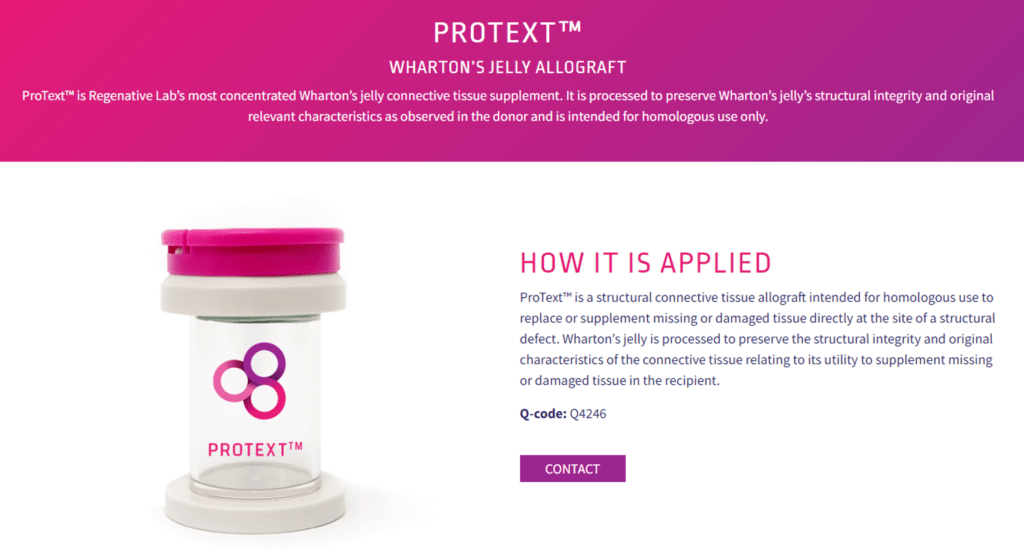
Note the wording above: “structural connective tissue allograft”, “homologous use”, “supplement missing or damaged tissue”, “structural integrity”, “relevant characteristics”, etc… This is the old lexicon of birth tissue manufacturers trying to stay away from getting nailed by the FDA. However, as of a few weeks ago, the FDA made very clear that the only homologous use medical indication it would accept for a Wharton’s Jelly allograft product like this one is now:
- “serving as a conduit (for umbilical cord)”
That is not what Regenative has listed here. That also kills all orthopedic use for these products, which is what Scott M. Martin, M.D is preaching above as the “Vice President of Clinical Development” during a company-promoted webinar.
Will Regenative Labs Survive?
In my opinion, I think most of these birth tissue companies will be dead in a year. That’s not only because CMS has them squarely in its sights, but also because of the dramatic changes in what the FDA will accept as a medical indication for these products. That loophole for orthopedic use has been so severely tightened, that the FDA is basically telegraphing its next moves here. Meaning IMHO it’s giving these companies rope to hang themselves, as the agency knows that all of them still need to promote these products in the orthopedic space to make sales. Hence, IMHO we should expect to see more Warning Letters and FDA visits for birth tissue vendors who are still advertising in this manner.
The upshot? You really can’t make this stuff up. The Regenative saga is one that’s super interesting to watch. I also suspect that it’s been very expensive for certain clinics that have had to endure clawbacks to the tune of hundreds of thousands to millions of dollars. Will Regenative’s new “hide in plain sight” strategy work? I don’t know, but I can say that there’s one rule in medicine that you must follow, “Don’t screw with Medicare!”

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
