Why Language from a New FDA Amniotic and Umbilical Cord Warning Letter Is a Big Deal
I’ve covered the stem cell wild west for years. One of the crazier things out was amniotic and umbilical cord tissue vendors claiming to sell “stem cell” products that don’t actually contain any live and functional stem cells. In addition, despite an FDA crackdown on these companies, there are still vendors selling this stuff with armies of sales reps hitting up doctors to purchase these magic products. The FDA this week hopefully put the final nail in this coffin with new language that should make anybody selling these products very nervous. Let’s dig in.
Birth Tissue Products
For the past decade, we’ve seen manufacturers and sales outfits selling birth tissues packaged in a vial. These have taken a few forms:
- Amniotic tissue
- Sheets
- Powdered finely chopped tissue
- Fluid
- Umbilical cord
- Blood
- Wharton’s Jelly
If you listen to the sales reps selling this stuff, these products contain loads of stem cells, which is a claim that our published research and that of others clearly showed is false (1-4). Another common claim is that these are all magical healing products. However, there is very little clinical data backing this up and even less comparing them to less risky and cheaper autologous orthobiologics like PRP.
FDA Crackdowns in this Space
Prior FDA crackdowns on birth tissue vendors have all focused on the concept that these are not 361 tissues that can be sold with a simple registration but unapproved 351 drugs. A biologic drug is called a 351 product in FDA lingo. However, most of the manufacturers have claimed that these products are instead 361 tissues. That category does not require clinical trials and FDA approval like a drug, but rather a 45-minute free online FDA registration without any approval process. Obviously, when you compare the tens to hundreds of millions of dollars to conduct clinical trials and get FDA approval for a new drug to the free online registration, you can see why anybody selling this stuff would be interested in the 361 tissue route.
The New FDA Warning
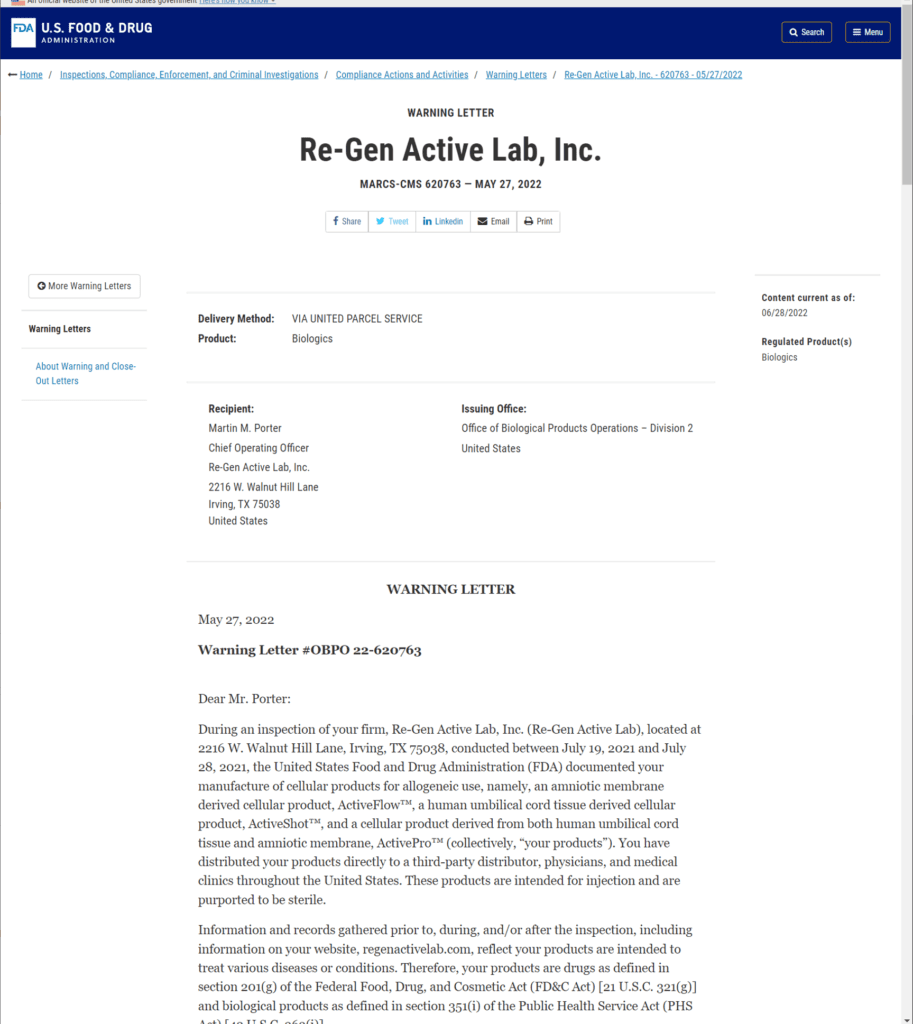
The FDA letters in this space have been regular, but they all suffer from being vague. What I mean is that the language in the letters sent to birth tissue vendors has all left openings for aggressive sales outfits to claim that while company X got busted, their advertising and sale of these products is instead legit. Well, that all just changed with the most recent FDA Warning Letter.
This letter was sent to a company called Regen Active Lab in Texas that sells both amniotic and umbilical cord products. It’s the result of an inspection that contains all sorts of FDA cGMP violations. However, the most significant change here is the dramatic shift in language from all other similar letters. Let’s look at the new language:
“Specifically, your products fail to meet the criterion in 21 CFR 1271.10(a)(2) that the HCT/Ps be “intended for homologous use only, as reflected by the labeling, advertising, or other indications of the manufacturer’s objective intent. Your products are not intended to perform the same basic function or functions of umbilical cord or amniotic membrane in the recipient as in the donor, such as serving as a conduit (for umbilical cord); or serving as a selective barrier for the movement of nutrients between the external and in utero environment, protecting the fetus from the surrounding maternal environment, and serving as a covering to enclose the fetus and retain fluid in utero (for amniotic membrane). Using your products for orthopedic diseases or conditions, for example, is not homologous use as defined in 21 CFR 1271.3(c).”
Why this all may seem complex, it’s really not. The FDA has a restriction on allogenic tissues for sale as a 361 in that they need to be used for the same purpose in the patient as they serve in the donor’s body. This is called “homologous use”. However, prior FDA letters have focused on vague terms like “cushioning”. For example, we know that the amniotic fluid can act as a cushion for the baby in the birth sac. However, by using that vague term, many of the manufacturers trying to fit their products into the 361 tissue mold have just parroted that language on their website. So while the sales reps sell their Wharton’s Jelly products as containing stem cells, their website and collateral focus on the idea that this jelly-like substance when injected into a knee will provide “cushioning”.
Now note that the FDA’s prior use of vague terms like “cushioning” has now changed to very specific statements like:
- “serving as a conduit (for umbilical cord)”
- “serving as a selective barrier for the movement of nutrients between the external and in utero environment”
- “serving as a covering to enclose the fetus and retain fluid in utero”
There is now no way that you can take these statements and apply these to orthopedic care. If that wasn’t enough, the FDA added:
- “Using your products for orthopedic diseases or conditions, for example, is not homologous use as defined in 21 CFR 1271.3(c).”
This is a Regulatory Thermonuclear Device Dropped into the Heart of the Birth Tissues Industry
This language change may not seem like much, but make no mistake, it’s epic. This eliminates all orthopedic marketing for these tissues, which is one of their biggest markets. Hence, I would expect many more of these Warning Letters. In addition, if I were a birth tissues company focusing on the orthopedic space, I would either fold up shop or raise tens of millions of dollars for a clinical trial.
The upshot? Birth tissue vendors can no longer make a credible claim that they can sell these products to doctors for orthopedic use by using vague terms like cushioning. That’s because the FDA has now VERY NARROWLY defined what homologous use is for these products. Expect many more FDA legal actions against birth tissue vendors to follow.
_____________________________________________________
References:
(1) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(2) Panero AJ, Hirahara AM, Andersen WJ, Rothenberg J, Fierro F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. Am J Sports Med. 2019 Apr;47(5):1230-1235. doi: 10.1177/0363546519829034. Epub 2019 Mar 7. PMID: 30844295.
(3) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(4) Berger DR, Centeno CJ, Kisiday JD, McIlwraith CW, Steinmetz NJ. Colony Forming Potential and Protein Composition of Commercial Umbilical Cord Allograft Products in Comparison With Autologous Orthobiologics. Am J Sports Med. 2021 Aug 16:3635465211031275. doi: 10.1177/03635465211031275. Epub ahead of print. PMID: 34398643.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.