Hip Arthritis Stem Cell Treatments: New Hip Registry Data
We’re in the process of replacing our 2013-2014 outcome data this next few months. We can do this, because unlike any other clinic treating orthopedic injuries with stem cells, we have developed a registry. What’s a registry and what does that have to do with hip arthritis stem cell treatments?
Almost all clinics treating orthopedic problems like hip arthritis don’t collect any data. A few decide after the treatment to send outcome questionnaires to patients, however this approach has it’s issues. First, without the patient routinely filling out a form to peg how he or she is doing before the procedure, there’s no way to compare any possible improvement or lack thereof to after the procedure. Second, sending questionnaires “retrospectively” (looking backwards) has issues, as there’s a strong possibility of bias (i.e. that patients who did well might be cherry picked to receive questionnaires or that patients might not accurately remember how they were doing prior to the procedure). That’s different from a prospective registry like ours that requires questionnaires before the procedure and at set time points. There is no other prospective registry for orthopedic stem cell care that we have identified-worldwide-outside of the Regenexx registry. Why?
Registries are expensive and resource intensive to maintain. As as example, first we had to customize existing Clinical Research Organization grade software. We also have three full time employees to physically contact patients via e-mail or phone when they fail to respond to the software. We then have a full time bio statistician with a medical degree to organize and analyze the data. This seems to be the main reason that despite big companies in this space making automated bedside centrifuges or smaller physician networks, we’re the only one collecting data at this level on a day to day basis. Which brings me to the new hip outcome information.
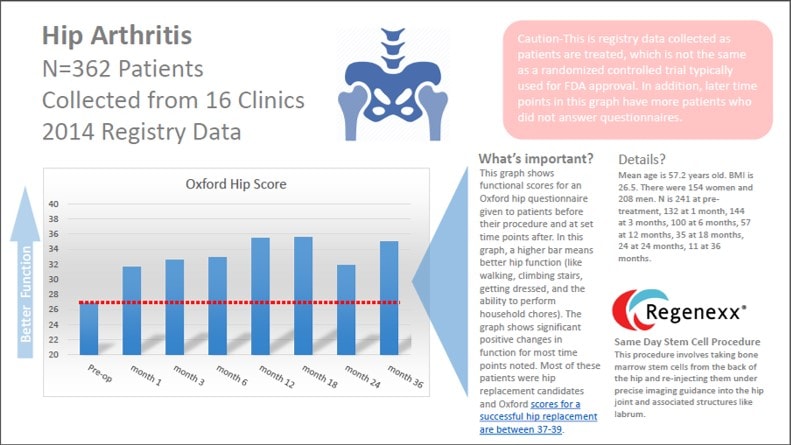
The hip arthritis stem cell data is on 362 patients tracked for up to three years. The questionnaire we used is a validated hip metric called the Oxford. It’s widely used in studies looking at the outcomes of hip replacement, so for a procedure that’s trying to avoid hip replacement in patients who are mostly hip replacement candidates, it’s a good choice. The questionnaire focuses on function, or what a patient can do. It asks questions about pain, limping, climbing stairs, getting dressed, moving around, and housework. The max score is 48 (best function) and most hip replacement patients score a 37-39 after their surgery. As you can see from above, our patients on average scored a 27 before the precise hip stem cell injection using the Regenexx protocol. The red line shows the starting place. In order to say that they had a “significant” improvement, their score would have to go up at least 5 points, and we beat that at almost all time points after the procedure. In fact, despite this being a minimally invasive stem cell injection versus a surgical amputation and insertion of prosthesis (hip replacement), our scores aren’t that far off from the 37-39 range. Having said that, as I discussed this week in the “What is Regenexx?” video, when compared head to head, while the Regenexx procedure for knee arthritis has about the same results as a knee replacement, stem cells used for hip arthritis under performs the results of a hip replacement (in this case slightly). This has remained a constant through out our many years of data collection.
The upshot? The new data on the Regenexx procedure for hip arthritis is encouraging. However, as we have seen in previous analyses, knee arthritis patients tend to out perform hip arthritis patients with stem cell procedures. Having said that, there’s no doubt that if you’re a responder, having a stem cell injection is a heck of a lot easier than getting a surgical amputation of a hip joint!
[Note: As always, click on the picture above to see a PDF copy of the infographic.]

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.