Our Amnio and Cord Stem Cell Scam Social Media Contest
This morning I awoke to this pic sent by Dr. Bashir in Miami. This is just the tip of the proverbial iceberg of an amniotic and cord blood sales industry that’s out of control. I’ve been thinking about running a social media contest for patients to educate everybody about the ridiculous claims being spouted by these companies and the chiropractic clinics spinning and conflating those claims. Hence, we’re going to allow patients to earn real prizes by identifying and calling out the scams.
What’s This Contest About?
We now have 40-50 companies that have popped up in the last 24 months, all selling birth tissues like amniotic membrane/fluid and or cord based products (like cord blood or Wharton’s Jelly). While these products have been used in neurosurgery and ophthalmology for decades, this was a niche market that could support just a few companies. So why the explosion of new companies? Some smart sales rep figured out that if he or she claimed these were “stem cell” products, they would sell like hotcakes. Or if they claimed they were growth factor rich products capable of orthopedic tissue repair, the same thing would happen. Then, clinics who didn’t have a clue what they were doing bought that sales song and dance and began telling patients that these were stem cell products. Is any of that true? Nope. These are best described as dead tissue products, in fact, they are regulated to ensure they are all dead tissue. Finally, we began to see chiropractic clinics who then began to spin incredible claims like you could grow new cartilage back using these dead products (see above Instagram post). You get the picture, it’s all one big massive scam. To learn more, see the video below:
Who is Axolotl Biologix?
My first reaction is, who cares? Meaning, there are so many new companies peddling amniotic and cord tissue after a 45 minute FDA registration that I can’t keep up. However, I can classify these companies as to the degree which they violate the law regarding their FDA 361 tissue registrations. What do I mean?
All these companies go through a quickie 45 minute FDA registration and aren’t required to get an actual FDA cellular drug approval. This is a big difference, as the former is free and has you up and running in a few hours, while the latter costs tens to hundreds of millions of dollars and takes 5-10 years. However, to stay on the right side of the law as a 361 tissue processor, they have to follow very strict rules. Step over the line and at the least you get a Warning Letter and at the most, you can be shut down or company executives can be prosecuted. Let’s just say that many companies bend or in some instances, macerate these rules in order to make sales.
What are The Rules for Amniotic Tissue Companies?
All the FDA permits these companies to say about these tissues is that they have a tissue to sell, here’s why they think it’s better, and what the FDA allows them to say about what it’s used for. So for an amnio fluid product, that means that they can say that they have an amniotic fluid product, here’s why they think how they process it is better, and that its use is for structural cushioning or as a wound cover. That’s it. Anything else is illegal and violates FDA law. So for example, if the company states that their product can repair tissue, that’s illegal. How do we know? It’s right on the FDA Tissue Reference Group (TRG) website:
OK with Quickie 361 Tissue Registration:
361 -A dehydrated chorioamniotic membrane in sheet form, used as a wound cover, is regulated solely under section 361 of the PHS Act and 21 CFR part 1271.
Illegal Biologic Drug without FDA Approval:
351 -A dehydrated chorioamniotic membrane used as a wound cover and to promote wound healing is not regulated solely under section 361 of the PHS Act, because wound healing is a nonhomologous use of chorioamniotic membrane.
To explain the above a bit further, the top entry labeled 361 just says that the amniotic product is used as a wound cover. That’s legal with the quickie registration. The bottom entry labeled 351 makes claims about the amniotic product healing wounds, hence that would need a full FDA approval with clinical trials and isn’t legal with a quickie 361 tissue registration. To learn more check out the video below:
Now that you know the lay of the land, let’s see where Axolotl Biologix falls in my new classification:
- Major Offenders: They have websites chock filled with illegal product claims
- Moderate Offenders: They take some chances on their websites by pushing the boundaries, but their online and printed material is mostly clean. However, sales reps or company representatives violate the law day in and day out to push product. This extends into their sales reps placing illegal product claims in writing.
- Minimal Offenders: They have clean websites, but their sales reps push the boundaries of the law.
- Clean companies: They have clean websites and clean sales reps.
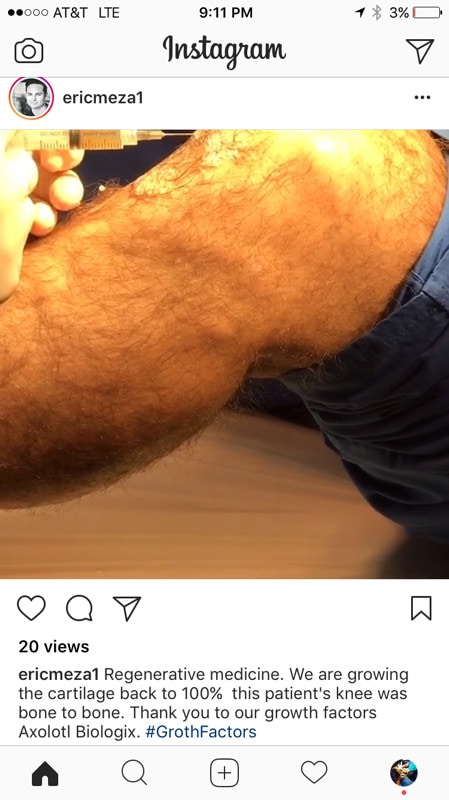
Given that Mr. Meza (who may be a sales rep or based on his other Instagram pics associated with the company) posted a picture claiming that this amniotic fluid product (i.e. he put it in writing) can heal cartilage, but since their website is clean, they are a Moderate Offender.
In fact, Mr. Meza actually posted this pic first:
Yikes! Note that he initially claimed that this product would regrow 100% of the cartilage back in this “bone on bone” knee. This is impossible in 2017 and has never been documented even with much more powerful culture expanded stem cell injections. Dr. Bashir called him out, so he took out the “100%” claim, but appears to be oblivious to the law, as his edited post (at the top of the page), is still illegal. So will Axolotl Biologix take action to correct this issue?
Is There Any Evidence that an Amniotic Fluid injection Heals Cartilage in Patients?
While there is some clinical data showing that bone marrow concentrate (BMC or a same-day stem cell injection) can help heal select cartilage lesions, there is no evidence that an amniotic fluid injection heals cartilage. In addition, there is zero evidence that it will make patients with “bone on bone” arthritis feel better. There is also zero published research on this particular product for knee arthritis. Finally, the IOF tested the growth factor content of amniotic products and found them to be growth factor poor compared to even a weak platelet-rich plasma shot, let alone a BMC injection.
Our Social Media Contest
Every week, someone is going to win a brand new, iPad or $1,000 off a Regenexx procedure. How does it work? 1. Identify, 2. document, 3. call it out.
Identify a statement online (web or social media) about amniotic fluid/membrane or cord blood that has no validity or truth or is illegal. These can be made by manufacturers, sales reps, or clinics. Examples include:
- They contain live and functional mesenchymal stem cells or are a “stem cell” product
- They can repair cartilage, tendons, ligaments, discs, nerves, or muscles
- The can regrow new cartilage in “bone on bone” knees and hips
Document it with a screenshot.
Call that statement out online. Examples are:
- Leave a comment that this statement is illegal or inaccurate
- If a comment isn’t possible, send us the screenshot so we can post it
- Link back to this post
- Use the hashtag #stemcellscam
How Do You Win?
The winner each week will be the patient who did the most work to tame this out of control industry. This will be determined by the reader who:
- Sent us the most screenshots
- Commented on the most social media posts (i.e. each inappropriate post commented on counts as one)
- Had the biggest impact. For example, someone with 500 followers who calls out 1 egregious post or website to their followers. Or someone with 100 followers who calls out 10.
Make sure you send us a link to your post calling out the problems. Use the hashtag #stemcellscam on Facebook, Twitter, Instagram, or Linkedin. Make sure you send your posts to: [email protected]
What Do You Win?
We will buy the weekly winner an iPad (mini or 9.7-inch size-64 GB) or the winner can get $1,000 off a Regenexx procedure of their choice (at participating sites). The contest begins this morning and I’ll name a winner every Saturday.
The upshot? This industry is out of control! Not only are many companies violating the law, but that’s perpetuating clinics that are scamming patients and telling them that they can regrow new knees from bone on bone arthritis. So help us stop the #stemcellscam and win some prizes!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.