Exosomes, Mary Kaye, and Pink Caddys…

If you thought the fake umbilical cord stem cell industry was bizarre, “you ain’t seen nothing yet”. Come with me as I take a tour down the rabbit hole of exosome marketing that may or may not come with a pink caddy. Let me explain…
What Are Exosomes?
Exosomes are tiny little packets of information shared between cells (1). This is a legitimate field of research study that’s just getting going. One day, once we figure a bunch of stuff out, exosomes may be used to help patients heal orthopedic injuries. However, right now, we don’t have a single clinical study showing that exosomes will help your knee, hip, shoulder, or back.
This statement from an actual exosome researcher says it all:
“Given the above, whatever the modification made on the exosomes or the choice of the cell-source, the problem of their safety remains. In fact, the challenge of research in the next few years probably will be to predict the exosomes’ behavior once injected. Little wonder all this can be achieved only after a careful and intense investigation on the characterization of the content of exosomes to be approved for clinical use.” (1)
That, of course, hasn’t stopped companies from selling exosome products. To learn more, see the video below:
Doctors as Exosome Marketers?
As you also know, I love to write about what I experience. So this past week I got a marketing email that said:
“Want to learn more about the science of mesenchymal stem cell Exosomes and how they can benefit your patients?
Email me and an MSC Exosome expert will follow up to answer all your questions.
Best Regards,
John L. Bender MD, MBA, FAAFP”
The email also included this diagram:
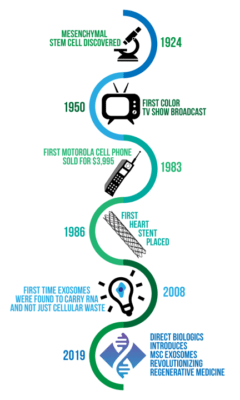
This picture claims that the Direct Biologics exosome product is equivalent to the discovery of the heart stent, cell phone, color TV, and the discovery of stem cells! I had never heard of Dr. Bender, a family doctor about an hour north of my office. Hence, I looked him up and called. He immediately broke into a sales pitch for exosomes and the one catchy line was “They’re all stem and no cell!” When I confronted him with the fact that the FDA had classified the exosomes he was selling as an unapproved drug product, he seemed surprised. He then told me that he and his partner, Ken Allen (a local spine surgeon who lost his medical license whose actual name is Kenneth Allen Pettine) were trying to conduct clinical studies. When I then discussed that no studies could be started without an FDA approval called an IND, he seemed really confused. Clearly, I was the first person who had discussed the actual regulatory pathway of exosome products with him.
Who Is Direct Biologics?
I know the company mentioned in Dr. Bender’s e-mail, called “Direct Biologics”. They have a snazzy website that says “Regenerative Healing”. It also says:
“The extensive intellectual property…for obtaining concentrated growth factors and exosomes from human mesenchymal stem cells… “
So they are selling exosomes and they also tell you that as a physician you can, “Increase your patient traffic using our team videos”
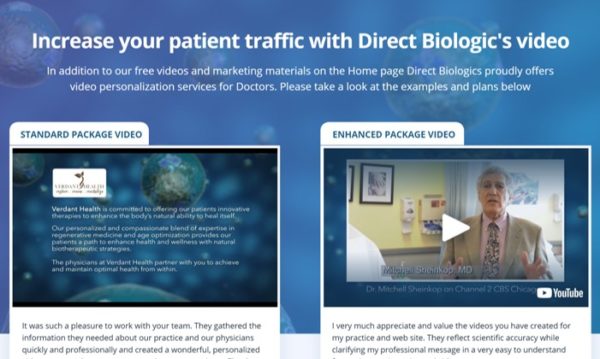
On this page, they tell you that they will make you (for about $40 a month) customized videos with your company’s logo and information. One video tells you that by offering exosomes you are offering “stem cell therapy”. The page makes claims about treatments for joints, spines, and urologic problems.
Is Any of this Legal?
I reached out to Direct Biologics numerous times for comment and they did not respond. I then consulted with a regulatory attorney on whether exosomes require a full FDA drug approval. The answer was a full “Yes”. Does direct biologics have a cell drug approval, NO. In particular, you also can’t place clinical applications on your website when you have a 361 tissue registration (which Direct Biologics has).
The FDA Letter on Direct Biologics
I actually asked the FDA TRIP program whether the Direct Biologics Exosome product was correctly registered as a tissue or an unapproved cell drug? This is what they said:
“As a general matter, exosomes for clinical use in humans are HCT/Ps regulated as biological products under section 351 of the Public Health Service Act and as drugs…To lawfully market a drug that is also a biological product, a valid biologics license must be in effect [42 U.S.C. 262(a)]….”
Hence, it’s an unapproved cellular drug.
Using Physician Surrogates for Marketing
I have never seen a physician surrogate marketing plan like this one. Meaning, if I didn’t read the initial promotional email carefully and I actually knew Dr. Bender, I would have assumed that I was really reading about the next best thing since sliced bread. I would have also assumed that Dr. Bender had carefully vetted the regulatory status of this product.
I told this story to a colleague at a medical conference and his first reaction was, “That sounds like Mary Kaye! Do they get a pink Cadillac? ” He said it, I didn’t.

The upshot? As I said if you thought the world of fake umbilical cord stem cell marketing world was strange, welcome to the world of exosome marketing! As I always say, you can’t make this stuff up!
__________________________________________
References:
(1) Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. Published 2019 Feb 15. doi: 10.1186/s13578-019-0282-2
(2) Campanella C, Caruso Bavisotto C, Logozzi M, et al. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int J Mol Sci. 2019;20(2):236. Published 2019 Jan 9. doi: 10.3390/ijms20020236

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.