Fake Clinical Trials in the Stem Cell Wild West?
It’s crazy out there; you’ve heard me say it time and time again. How crazy? We’ve had many clinics offering fake clinical trials for some time, but the increasing sophistication of these offerings has even caused me to do a double take. So this morning we’ll delve into what’s going on.
What Is a Clinical Trial?
In medicine and drug development, the idea of a clinical trial sounds great. Most patients would equate that language with the type of scientific sophistication that makes them feel confident in whether a product or therapy works. But what does that phrase really mean?
A clinical trial is a scientific study performed on a drug, device, or procedure. The purpose is to see if medical care is safe and effective. The type of clinical trial that patients see most often in the news has a few key elements:
- There is usually a spiffy acronym as a name. This may be something powerful, like IMPACT, SUSTAIN, or HEAL.
- There are strict inclusion and exclusion criteria for who can enter the trial. Meaning, the patient may only have x, y, and z on their tests and can’t have a, b, or c. For example, the patient can have knee arthritis with no more than a KL grade-2 X-ray and can’t have rheumatoid arthritis and can’t walk 100 yards without difficulty.
- The study is no cost to the patient. This just usually means that the study-related care is free.
- The patient is randomized to either receive the therapy being tested or another therapy or a placebo.
- Everyone gets the same treatment.
- Outcomes and complications are closely tracked.
So you get the idea. A narrow group of patients who all have the same condition in the same or similar stages and who can’t have other conditions are treated at no cost and randomized to one treatment or another while their outcomes and complications are closely tracked.
Tracking Outcomes vs a Clinical Trial
As you can see above, there are quite a few rules involved in a clinical trial, especially one that is a higher-level study (meaning that it’s randomized). However, now let’s compare and contrast that set of expectations to what many stem cell clinics claim is a clinical trial, but it’s really not. As you’ll see, there are stark differences. Here I’ll start with explaining how care can be delivered while the results are tracked, which is what I call “Tracking Outcomes.”
Tracking outcomes while you treat patients is a time-honored tradition in medicine that’s common in investigational care or even for medical care that’s more traditional. In orthopedics and cancer care, for example, there are national registries usually run by professional organizations that track outcomes for many physician practices. In orthopedics, for instance, there are national knee and hip replacement registries.
The big difference between tracking outcomes and a clinical trial are as follows:
- Since this isn’t a formal study, there is no spiffy acronym.
- There are no inclusion or exclusion criteria, meaning that pretty much anybody with the diagnosis gets tracked.
- Patients are usually charged for care.
- There is no randomization as everybody gets care.
- Outcomes and complications can be anywhere from closely to loosely tracked.
Hence, tracking outcomes is VERY different from a clinical trial. That isn’t to say that it’s not an important endeavor, especially when a registry is involved, meaning there are many scientific publications out there that help advance medicine that derive from registry data.
Confusing Patients by Calling Tracking Outcomes a Clinical Trial
I’ve seen for years that certain clinics describe the process of collecting outcomes in a way that gives the impression of a clinical trial. This morning I’ll cover both an example that’s a little deceiving, in my opinion, and one that’s very deceiving. Let’s review.
The first is an example from a chain of fat stem cell clinics that has this page listed under a “Currently Studying” tab:
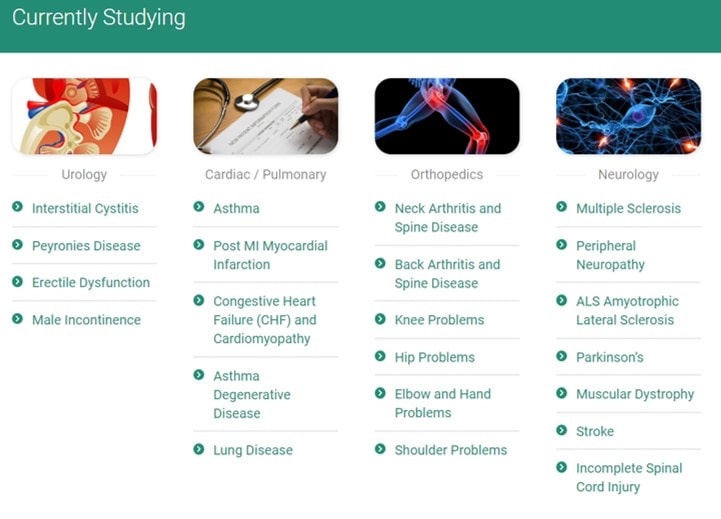
First, you certainly get the impression that these are individual clinical trials. However, they are not clinical trials, but, instead, only a clinical advertisement for treatment where there is some effort to track outcomes. However, this is not the worst example of what’s out there.
Now let’s go to the other end of the extreme. This is from a clinic in Florida:

What you see here are multiple fancy trial names. We have SCOTS2, NEST, ACIST, and SciExVR. You can even find these trials listed on www.clinicaltrials.gov. However, since anybody can list anything on www.clinicaltrials.gov and that system is often abused by stem cell clinics, that doesn’t tell us much. This is an interesting article on that topic.
Hence, any reasonable person would think these were cell-drug trials. However, looking under the hood more closely is where that theory comes head to head with reality. These are, in fact, merely cases where the doctors are charging for treatments and tracking outcomes. For example, take the NEST “trial.” The text reads as follows:
“We are interested in the numerous neurologic diseases and degenerations that are present. The major categories include Chronic Stroke (CVA), Parkinson’s Disease, Multiple Sclerosis ( RR, PP, PR, SP), Traumatic Brain Injury (TBI), Peripheral Neuropathy (including Diabetic Neuropathy) but diseases are considered on a case by case basis. Typically patients receive BMSC intravenously and intranasally.”
That’s an incredible number of conditions for a clinical trial! This is what the www.clinicaltrials.gov page lists as the conditions treated:
Neurologic Disorders
Nervous System Diseases
Neurodegenerative Diseases
Neurological Disorders
Stroke
Traumatic Brain Injury
Cadasil
Chronic Traumatic Encephalopathy
Cerebral Infarction
Cerebral Ischemia
Cerebral Stroke
Cerebral Hemorrhage
Parkinson
Multi-System Degeneration
MSA – Multiple System Atrophy
Progressive Supranuclear Palsy
ALS
Amyotrophic Lateral Sclerosis
Neuropathy
Diabetic Neuropathies
Huh? A true clinical trial would study only one diagnosis at a time. Meaning, like multiple sclerosis (MS). Then a clinical trial would focus still further and study only one type of MS, like “relapsing remitting.” However, here we have four different subtypes of that disease. Add in that they will also consider other neurodegenerative diseases on a case-by-case basis and list almost every neurodegenerative disease known to man in their “inclusion criteria,” and what we have here is definitely not a clinical trial.
A clinical trial also generally requires that the treatments being studied are free of charge. Now that’s not always the case as some trials allow patients to pay. This treatment is in fact almost twenty thousand dollars. So add that together with these hugely broad inclusion criteria and we definitely have a treatment where outcomes are tracked and not a clinical trial.
The Ethics of Naming Outcomes Tracking as a Clinical Trial
I’m not the only one to call this website out, as Paul Knoepfler on his blog also noted this issue. So what are the ethics of naming paid treatment where you’re collecting outcomes like it’s a drug trial? For me as a physician leader in this space, this crosses an ethical line. Why? Patients will look at this and believe that a true drug trial is taking place. In addition, since there are some actual clinical trials with strict inclusion and exclusion criteria that allow patients to pay for therapy, they will more likely than not be confused here. Meaning, most people won’t know that this is more of an advertising strategy than it is a scientific endeavor.
Having said all of that, isn’t it better to collect outcomes than not? Absolutely. The problem comes in when you try to make people believe they’re getting X, but you’re actually delivering Y. In this case, that the patient is entered into a clinical trial as if they are testing a drug on a specific disease, and instead they’re just getting a paid treatment. Let’s review how we have handled all of this stuff.
What Do We Do at Regenexx?
At Regenexx we have always tracked our patients who consent in a rigorous registry. That’s very rare, especially since we’ve been doing it for 14 years and have collected more data on orthopedic stem cell treatments than anyone else on Planet Earth. However, we don’t try to make people believe that they are part of a clinical trial when they are paying for that care. Instead, they know that they are part of investigative care and their outcomes will be tracked as long as they consent to have them tracked. They also know that this data may be used for publication (without their name being used).
We also have multiple true randomized controlled trials where the patients get free care. Given that these all have strict inclusion and exclusion criteria, it would be ethical to name them like drug trials. For example, our just-completed and published knee arthritis trial could have been named “Knee OSteoarthritis Trial,” or KOST. Or our existing shoulder rotator cuff trial could be named “ROtator Cuff Stem cell therapy,” or ROCS! Or some other heroic sounding name that when you read it on the page you hear either trumpets blaring or angels singing. Who knows, maybe we’ll come up with names.
The upshot? It’s always interesting to me to see how the stem cell wild west is evolving. This is a new one, where we have clinics that are tracking outcomes naming paid therapies like drug trials to provide an extra veneer of credibility to sell more treatments. I’m not sure what’s next, but it is certainly amusing at times watching the creativity. The problem? When the creativity outstrips reality.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.