What Would the Research Base of Orthopedic Stem Cell Injections Be Like if Regenexx Didn’t Exist?
It’s a Wonderful Life is a Christmas classic. In this movie, the main character gets in a rut and questions whether he should have ever been born. He’s then met by a ne’er-do-well angel who grants his wish and shows him a world where his town has gone to pot (or Potter). In the end, he realizes the huge impact he’s had on his little world, and everyone lives happily ever after. This week, when I posted our newest low-back-disc research paper, most physicians were grateful to have more data on the topic, but one sales rep (who wasn’t happy about our data from months back that demonstrated that the device he sells didn’t work well) was more upset by the paper than excited. Yesterday, as I went about my day, this got me thinking. What would the world of orthopedic stem cell injections be like if we had never existed?
At Regenexx, we’ve always spent generously of our time and resources on tracking patients and studying things in the lab. This makes sense to me, given that we invented orthopedic stem cell injections to treat common things, like knee and hip arthritis and degenerating low-back discs. However, a lot of people have gotten quite a ride on our research coattails. Meaning, the registry-based research that we have been able to publish has formed the basis for thousands of providers and companies to begin offering stem-cell-injection procedures and devices worldwide. Don’t believe it? Let’s take a look.
What Was Published Before Regenexx?
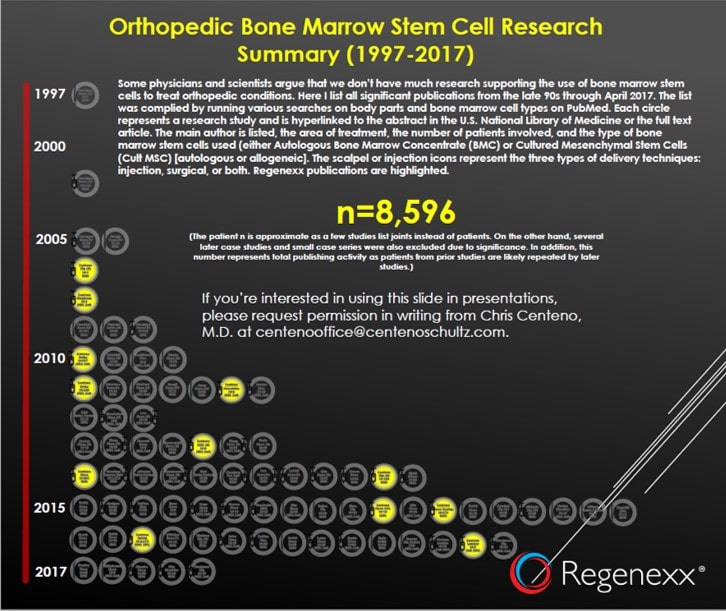
All that existed before our first publications in 2006/2007 on using culture-expanded stem cells and bone marrow concentrate to treat knee and hip arthritis were papers focused on treating osteonecrosis. These used bone marrow concentrate in primarily surgical procedures (CORE decompression). Given that I’ll be using my April 2017 Orthopedic Bone Marrow Stem Cell Research Summary, there’s a copy below at the thumbnail. If you want to check anything I’m writing, just click on the image and you’ll get the PDF with active links to the studies in each circle icon.
What Impact Did Regenexx Research Have on the Early Years?
For the earliest physicians getting involved in using stem cells for orthopedic purposes in 2009–10, this is all they could hang their hat on from a research perspective if someone called them on the carpet for injecting knee and hip joints with stem cells:
- Our knee and hip osteoarthritis case reports
- Our first safety paper on 227 patients who received primarily injections of the hip, knee, shoulder, and low-back discs
- Various fracture nonunion and osteonecrosis papers that were mostly surgical, primarily by Hernigou, and a surgical paper on ankle arthritis
So the only evidence base that existed that “stem cells” were safe after being injected into joints was that n=227 safety paper published by Regenexx. That paper featured a coauthor from Harvard. So if you’re one of the very few physicians who began using stem cells prior to 2011, you’re welcome.
After Our First Big Safety Paper
In the 2011–2012 time frame, this field went from a handful of physicians offering orthopedic stem cell treatment to dozens who jumped on board. In 2011 we published our larger paper on safety with some efficacy data, now up to 339 patients. So if you got called on the proverbial carpet back then for injecting stem cells into joints, tendons, or discs, what could you give your medical board to make sure you didn’t lose your license?
- Our two safety papers of n=227 and n=339 which also had some clinical efficacy data
- Two small case reports on low-back disc
- Hernigou’s and others’ papers on fracture nonunion and osteonecrosis, which was primarily surgical data (none of which involved treating joints)
- A few small- to medium-sized case series, primarily out of Japan, on surgical treatments for knee
Again, if you began injecting stem cells into knees and hips to treat osteoarthritis in 2011–2012, we made up about two-thirds of the safety data you would have been forced to use to defend your procedures.
What if Regenexx Hadn’t Published Everything Up to the Present?
As of April of 2017, our patients made up approximately 50% of all patients who had their results and complications published. So right off the bat, if Regenexx didn’t exist, the research base for this field would be far behind its current state.
Let’s look more closely at how the field would be different for each procedure now being commonly performed:
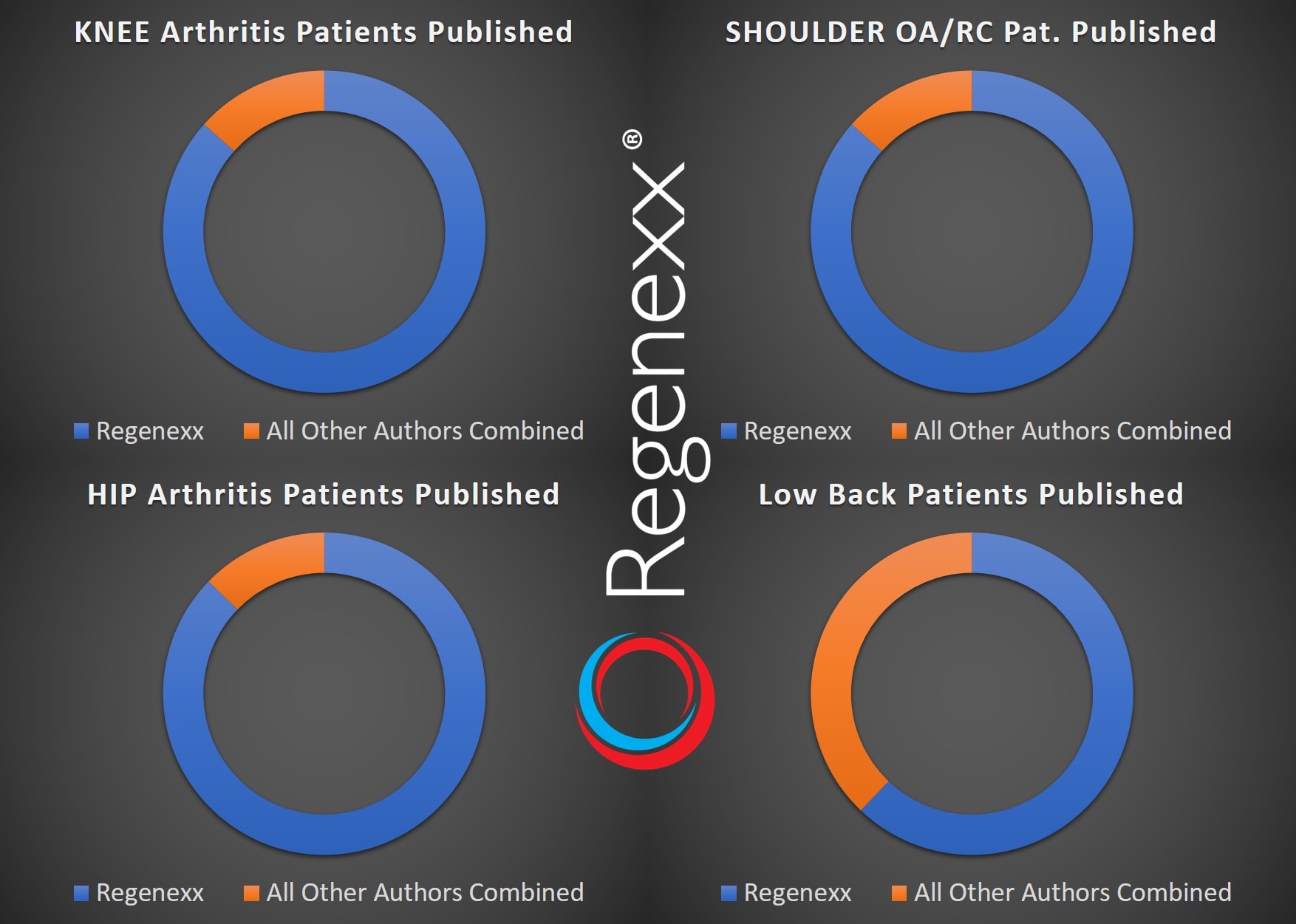
- Knee arthritis injections: We published data on 337 procedures in our 2011 cultured-stem-cell paper (safety and efficacy), 840 patients in our knee bone-marrow-concentrate paper (safety and efficacy), an additional several hundred patients in our recent largest- of-its-kind safety paper, and an additional 10 in our ACL paper. Hence, we’ve published on about 1,300 patients who had their joints injected with stem cells for arthritis. How many patients are there in all other studies by all other authors on stem cell injections for knee arthritis (excluding the surgical studies)? Only 201 patients by my count this morning.
- Hip arthritis injections: We published on 192 procedures in our 2011 safety paper, on 216 procedures on 196 patients in our 2014 hip paper, and on approximately another hundred patients in our recent safety paper. That’s about 500 Regenexx hip arthritis patients. How many patients have had their results published outside of Regenexx as of April of this year who had hip injections for arthritis? Only 73 patients!
- Shoulder arthritis and rotator cuff: In our 2011 paper, we published on 43 shoulder procedures; in our 2015 shoulder paper, we reported on 115 procedures in 102 patients; and in our recent safety paper, on about another 30 patients. That leaves approximately 200 shoulder stem cell injections that we have published. As of April of this year, nobody had published anything on shoulder injections used to treat arthritis or rotator cuff tears. The closest you could get would be Hernigou’s 45 shoulder patients, but they all had surgery before their injections, so the purpose of that paper wasn’t to use injections alone. (I added the 45 patients from Herigou in the graph below.)
- Low-Back Injections: Our 2011 paper published on 16 patients, our recent large safety paper on 59 patients, and our just-published clinical outcomes paper on 33 patients. Given that there is significant overlap, to date, in these data sets (all lumbar injection sites versus just the disc) and we’re using everything up to April 2017, we have published on 59. Everybody else combined? 36 patients to date.
Here is what these summaries look like in graph form:
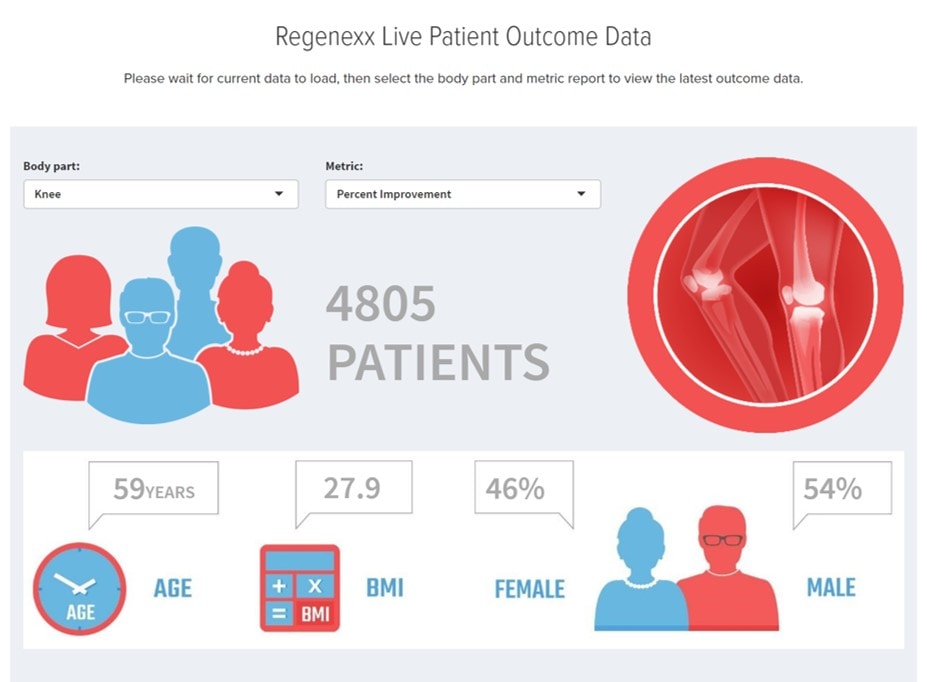
We also have far more data online, which includes the ten thousand patients whose stem-cell-treatment registry data is shared and updated monthly on our website:
If you are a physician performing stem cell injections for the knee, hip, shoulder, or low back, if Regenexx didn’t exist, as you can see above, you would literally be operating in a world where not much was known about what you’re doing. If we adjust for the fact that many of the injection-based studies used above were with culture-expanded mesenchymal stem cells and not bone marrow concentrate (same-day procedure), there’s even less evidence outside of Regenexx. Bottom line? You would have a hard time defending what you’re doing.
If you’re a patient, the multitude of offerings for stem cells you see that now exists, in large part, because Phillipe Hernigou paved the way in the ’90s with bone procedures and because we paved the way for injection-based procedures in commonly injured and arthritic joints. It’s just that simple.
If you’re an orthopedic sales rep selling stem-cell-related devices, thank your lucky stars we exist. If we didn’t, I expect your sales would be a fraction of what they are right now, and most of you would have never heard of this space by 2017. So while you might hate that our independent lab tests don’t always agree with your marketing collateral, your current job literally depends on us being here.
The upshot? As of April of 2017, Regenexx research has dominated what we know about stem-cell-injection procedures for commonly treated orthopedic problems. If we had never existed, the amount we know about the safety and efficacy of these procedures would be a minuscule fraction of what it is today.

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.