The Initial Marrow Cellutions Device Results

As promised, here are the Marrow Cellution (MC) test results. To review, this is a special trocar that purports to draw many more stem cells from the bone marrow. It’s shown above on the bottom versus a standard trocar on the top. This was the first patient yesterday, so this data in not conclusive. Having said that, I learned some interesting things about the device, some good and some not so good.
For more details on the actual test we’re performing, see yesterday’s Marrow Cellutions blog.
The Reason to Test This Device
As you can see above, the Marrow Cellutions device is on the bottom (listed at $700). I paid $600 for this device as I bought it through a physician who was a repeat customer who gets a “deal.” I’ve seen it quoted to our group and others from $700 to as much as $1,900. It’s widely sold through resellers, hence the wild price differences. Above, in the picture, is a standard Jamshidi needle that costs about $35 and is the most common instrument type used to draw bone marrow aspirate.
Because of its expense and the claims that it can pull out some 5X more stem cells from the bone marrow, its manufacturer recommends that you don’t need to concentrate this aspirate. This is quite different as, normally, physicians will take a larger volume aspirate and then concentrate the stem cell fraction. This device takes less marrow and the recommendation is to just inject it “as is.”
First Impressions
Man, this thing is big! As you can see above, it’s about 2X+ the size of the standard trocar and weighs 3–4 times as much in the hand. This can make it a bit unwieldy for patient use, but I’m sure if you used it every day you would get used to the size and weight.
The company claims that it’s dramatically faster to use, thus saving the doctor time. However, I can’t see how that would ever be the case. There are just more moving parts and steps with MC that you don’t find with a Jamshidi trocar. You need to first place the main device, then change out to a blunt-tip stylet, then advance the stylet, then remove it and replace that with an aspiration needle, then advance that, then turn a screw that descends toward the patient’s skin (skin screw), then remove a stylet, then attach a syringe, then aspirate, then rotate the handle, aspirate, and repeat. The video is below.
There are about half that many steps when you’re using a Jamshidi needle in the same way. Hence, while this could be similar in doctor time to some marrow-draw techniques, it actually takes longer than using a Jamshidi needle with the same protocol. As with the latter, you simply place the needle, remove the stylet, and rotate/aspirate as you remove the device. Done.
Performing a BMA Procedure with Marrow Cellutions
The MC device is designed for what I call the single-site, deep-dive BMA. Regrettably, we have many types of BMA procedures being performed. Everything from a physician placing a trocar and drawing out a single large volume of marrow aspirate (which dramatically reduces the number of stem cells but makes it easier for the doctor) to placing the trocar deeper and aspirating periodically as you retract the device to multisite lower-volume draws. The MC manufacturer advises that you perform a single-site draw and aspirate 8 ml total as you retract. To get the device to back out, you turn the handle 360 degrees after a screw has been turned and placed against the body. Essentially, the device is pushing against that skin screw to back out the trocar to a different spot so that a bit more marrow aspirate can be taken (see video above).
Getting Screwed
Most of these MC draws are either performed blind or under ultrasound guidance. The downside of both techniques is that you have no idea what’s happening with the aspiration needle deep in the body. It’s supposed to be retracting a bit every time you turn the handle so that you can aspirate from a different spot. However, when I used fluoroscopy (real-time X-ray) to check its position, it wasn’t budging despite turning the handle a few times. The issue was that even though I thought that I had the skin screw tight against the patient, it wasn’t nearly tight enough. In fact, thankfully I had this area numbed as getting it tight enough so that the aspiration needle would move meant tightening it against the patient’s skin a bit more than I felt comfortable. While I finally got the needle to start backing out on the fluoro images, it brought up a concerning point. I would bet that many users of this device have their first few aspiration pulls coming from the same spot and they have no way of knowing that because they aren’t using X-ray to guide the procedure. I would also bet that this effect is worse in obese patients (this patient wasn’t obese). The good news is that I practice in the thinnest state in the nation. However, physicians in certain parts of the country should test this device using fluoro to get a sense of what it takes to get it to work properly.
A Clotty Problem
Our usual method for drawing marrow is to preinject each bone site with a small amount of heparin anticoagulant. This is a trick we’ve learned to ensure that the clotting cascade doesn’t get started before the marrow aspirate is drawn. Why? If it does, you get clots in the aspirate, which robs stem cells in the final patient injection and can make reinjecting through small needles difficult. Since we were following the protocol that was published by the manufacturer of the Marrow Cellutions device, I didn’t pre-heparinize, but instead, I did what everyone else does: I coated the device in heparin. Regrettably, we paid for this deviation of our normal protocol as we had clots in both the side we did with the MC device and the standard Jamshidi side.
From communicating with a regular user of the MC device, this is an issue he regularly sees as well. This is a problem as clots generally can’t be reinjected and end up robbing stem cells that will stay in the syringe or being discarded when they foul the needle. In essence, clots rob stem cells from the patient. Hence, in the next patient in our MC trial, we won’t make this mistake and will need to pre-heparinize.
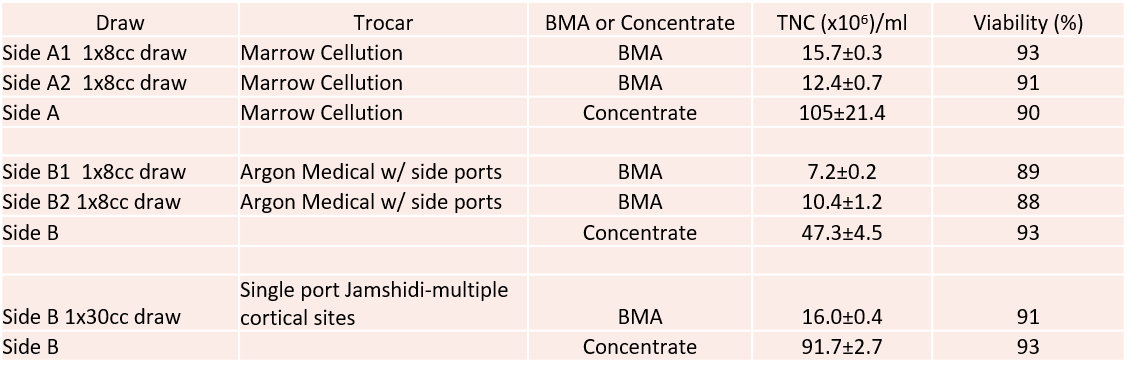
The Stem Cell Counts—$700 vs. $35
So how did the MC device do versus a $35 trocar? Reasonably well when used in the same way, not so great when used in the way we normally perform a draw. Per unit volume counts of the TNC (total cells in the sample) are below:
So what’s shown? The good? The Marrow Cellutions trocar beat the less expensive side-ported device used in the same way by pulling about 50% more total cells. The bad? Once those cells were concentrated using our usual protocol, this beat the Marrow Cellutions number about 300%. Hence, the claim that the MC device gets so many cells in the draw that concentrating the aspirate is not needed didn’t hold up in this test.
What was most fascinating was the third draw I did in this patient. This was our standard draw using a single-port trocar where I went to multiple sites and drew small volumes. Time wise it took about half the time of using the MC, but that may come out in the wash with more MC experience. However, despite this being the third draw from the same area (the MC had already been through this side of the pelvis twice), this yielded >600% more cells per ml than the MC device. More importantly, concentrating these cells got about the same amount as concentrating the BMA drawn with the MC device.
The Knee Dosing Problem
We know based on our research study published in 2015 that we need a TNC count of about 400M to get the best result in an arthritis patient. So if we take the best MC device draw of 15M cells per cc and multiply times 8, then we only get to 120M cells—far below that threshold. Based on the numbers above, If we had used our normal draw technique on a man this size (multiple low-volume cortical sites and a draw of 90–120 ml), after concentration, we would have had enough cells for a good knee arthritis treatment. If we had used the MC device and performed multiple passes (i.e., the A1 and A2 in the table above) and then concentrated those cells, we also would have had enough cells.
Disclaimers
First, I report TNCC only here (total cells and not stem cells, which are a fraction of the number shown here). So we still have a stem cell count pending that will take about a week to form colonies in culture (CFUs). Second, nothing is settled by a single-patient side-by-side test, so more side-by-side comparisons will be done. I’ll compare this device on at least two more patients before I decide if the results are compelling enough to add more patients to this trial.
The upshot? So is a trocar that costs 20 times as much worth the price? Not based on this early test. In fact, our standard way of drawing marrow perfected over 12 years and thousands of draws did just as well. Is the idea that you can use the product of the MC device draw without concentration valid? Again, not using this data. So we’ll have to see how our next two other patients fare, but so far, this came out about the same as the last expensive bone-marrow-draw device that we tested (Marrow Miner).

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.