INVITrx “Stem Cell” Review II: Like Buying a New Car?
It’s almost a full-time job keeping up with the bait-and-switch fraud that is the umbilical cord “stem cell” industry. While this is one product called INVITrx that I had blogged on before, now the company seems to have altered its sales pitch. Let’s dig in.
The Umbilical Cord “Stem Cell” Scam
Companies that take umbilical cords from live births and then try to isolate the cells and freeze this stuff have had a heck of a ride these past few years. By claiming that this is a stem cell product that has millions of young and healthy stem cells, they have been able to sell this stuff to gullible physicians, chiropractors, naturopaths, midlevels, and acupuncturists for top dollar. How much? If you sell all of the parts of the live birth—amnion, amniotic fluid, umbilical cord blood, and Wharton’s Jelly—you’re talking about a cool million per delivery.
Of course, the physicians should know better, but their lack of training and experience in basic cell biology dooms them to believe the stuff told to them by sales reps. The chiropractors and other alternative health practitioners don’t have the science backgrounds to digest any of this information. Hence, you have the blind selling the blind who then sell this lie to patients.
Why is this all a scam? IT’S ALL DEAD TISSUE!!! Meaning there are no live and functional stem cells in any of it. We’ve done this testing as has Lisa Fortier at Cornell. Listen to a short snippet of her podcast, or read her abstract.
My First Review of INVITrx
My first review of the INVITrx umbilical cord blood product was back in September of 2017, and at that point, I pointed out poor viability and flow cytometry testing that was performed incorrectly to identify mesenchymal stem cells (MSCs), which the manufacturer claimed to have in spades in its product. To see my video on that topic, see below:
One of the big issues was that the minimal number of cell surface markers that are required to determine if MSCs are present weren’t tested. Then, out of the blue, a colleague sent me a recent presentation on INVITrx. So I went back to their website to see if they had changed things. Suffice it to say that I chuckled as hard after looking at the new data on their website as I did when I first looked at the first white paper produced by the company. So let’s dig into the company and then the new information.
INVITrx and the FDA
The company behind INVITrx had an FDA Warning Letter sent in 2017 that discussed a variety of claims for its cosmetic products, many of these stem cell based. So did the company clean up its act? Meaning, its simple 45-minute registration would not permit any claims about live stem cells. I went to their website to see and found this PDF:

Flow Cytometry and Cars
My wife’s car just had a spectacular engine failure off warranty. The cost to fix it was insane, so it was time to shop for a new car. Which is what likely gave me the idea to teach you about flow cytometry by using car buying. The video above can get you there quickly, or feel free to read on.
When you buy a car, you’re looking for certain things. Exterior and interior colors can be important as can make, year, model, and engine. In fact, you can write the description of a specific car you’re looking for with a string of descriptors like a red 2016 Porsche Cayenne with a black interior with a V6. However, what would happen if you called a used car place asking for that specific car and here’s what the sales guy told you:
- We have 34 red cars here.
- We have 62 cars on the lot with a black interior.
- We have 6 cars that are Porsches.
- We have 2 cars that are the Cayenne model.
- We have 162 cars with 4 doors.
- We have 122 cars with a V6 engine.
- We have 32 cars on the lot that are a 2016.
Do you see what just happened? Instead of identifying your specific car by using all of your descriptors that describe it, the salesman just told you how many cars met each descriptor. At this point, you would be apoplectic, right? Well, that’s what INVITrx does in the above graphic. Let me explain.
To describe a mesenchymal stem cell, FACT (an international cell-therapy-standards group) puts out a list of cell surface markers that have to be there and be absent in order to make that call. Why? Because there isn’t a single cell surface marker that will do the job, just as there isn’t a single descriptor that will describe the Porsche Cayenne you’re looking for. With that little bit of knowledge, now let’s look at the flow cytometry data reported above on the INVITrx umbilical cord blood product:
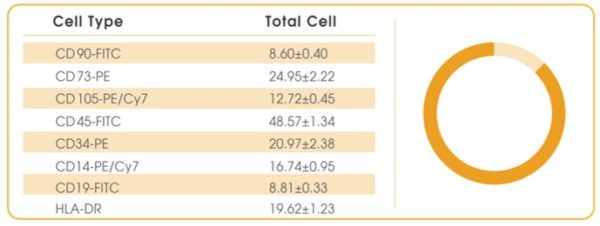
So on the left, we see a mislabeled column called “Cell Type.” These are, in fact, cell surface markers (e.g., CD90, CD73, and CD105) with their respective color dyes listed (e.g., FITC, PE, Cy7). The author of the table believes that these describe specific cells, and in a few instances, that would be somewhat right, but in most it’s mostly wrong. Meaning that the cells in your body often have many more than one marker, and most of these aren’t specific for any cell type. For example, CD90 is found on thymocytes, prothymocytes, neurons, mesenchymal stem cells, hematopoietic stem cells, NK cells, endothelium, renal glomerular mesangial cells, circulating metastatic melanoma cells, follicular dendritic cells, fibroblasts, and myofibroblasts. These are the same as the car descriptors above, like make, model, engine, color, and year. In the same way, the descriptor “red exterior” describes lots of different cars.
On the right is the number of cells in this tested sample that had that cell surface marker. Now what’s bizarre here is that there’s actually one thing missing. In flow cytometry, trying to identify mesenchymal stem cells (MSCs), some of the markers have to be present and some absent. These are usually designated with a (+) if they are there and a (-) if they’re absent. None of that seems to be here, so right off the bat, it’s impossible to use this data to determine if there are any MSCs in this sample. This is the same with describing cars, right? I want a red one and not a white one, and I like the V6 engine and not the V8. For a car, you could write this something like this: Red+, Black-, V6+, V8-. The positive sign says that this attribute is found, and the negative sign says it’s not found. It’s the same with these cell surface markers. To identify a single MSC with flow cytometry, you would need the following:
CD105+, CD73+, CD90+, CD45-, CD34-, CD14-, CD19-, HLA-DR-
Then you would list the number of cells that had CD105, CD90, and CD73 present while also having CD45, CD34, CD14, CD19, and HLA-DR absent. That’s not what happened here in the table above. In fact, this is like the used car salesman who told you he had so many red cars and so many with V6 engines and never told you if he had the car you wanted.
In summary, this flow cytometry data is useless in determining if there are MSCs in the INVITrx product.
Other Reasons Why the INVITrx Data Is Not Complete
In order to actually tell if there were living and functional MSCs in this sample, you would not only need all of the cell surface markers present and absent as described above, but you would also need to grow cells out in culture and perform other tests. This is per those same FACT guidelines. See my video below for more information:
So this information looks great on paper to the uneducated, but now that you know what should be there, you can see it’s not that helpful in actually supporting the manufacturer’s statement that this product has live and functional MSCs.
The upshot? The next time you see one of these flow cytometry reports issued by an umbilical-cord-blood manufacturer, just think about buying a specific car. You need all of the descriptors to get the car you want, and, in the same way, you need certain cell surface markers present and absent to get the cells you want. They also have to survive to be able to form colonies in culture and pass other tests, but none of these manufacturers have ever gotten that far!

If you have questions or comments about this blog post, please email us at [email protected]
NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.