How Many Times Can You Violate the FDCA in One Marketing E-mail?
I’ve blogged before about the explosion of amniotic and cord vendors illegally claiming live stem cells in their products. It would be easy to look the other way if any of this were verifiably true, but near as I can tell, it’s all incomplete data designed to push product down the throats of gullible physicians. In fact, because most physicians have taken the time to educate themselves, we primarily tend to see acupuncturists and chiropractors who seem to be the ones biting the magic stem cell messaging. This morning’s ridiculous e-mail comes from a product called “StemShot,” which likely wins my “Most Violations of the FDCA in a Single Communication” award.
Understanding Umbilical Cord Products
I’ve blogged on this stuff many times, so it’s best to use my pre-existing videos to understand how StemShot violates the FDCA and is more likely than not selling a dead-tissue product. As far as violating federal drug laws, watch the video below:
As far as whether these products have any live cells or are dead stem cell products, watch this video:
Let’s Look at the E-mail Advertisement
I was forwarded this e-mail by several colleagues this week as it seems to have been part of an e-mail blast that was sent out to many physicians. The e-mail ad is hawking StemShot and StemVive, manufactured by the Utah Cord Bank.
The bizarre thing here is that this ad wasn’t sent by the company that makes StemShot, but an orthopedic sales rep called Apex Biologx. This is concerning, as if the manufacturers decide to break the law, then a sales rep should distance themselves from that event. By amplifying the messaging here, Apex is making itself liable under 21 USC 331(c). Meaning that Apex, by selling and promoting a misbranded and adulterated drug product, is liable and can be prosecuted right along with the manufacturer.
The Claims Made About the Products
First, realize that the FDA is a claims made regulatory system. Meaning, if you are selling a vial full of dead or dying cells and you claim that they are alive and can heal tissue, just the claim is enough to change your regulatory status from a tissue to a drug. Whether the claim is actually true or not is typically something for the Federal Trade Commission (FTC) to address pursuant to its decades-old Memorandum of Understanding with the FDA. So while I highly doubt that these products contain live and viable mesenchymal stem cells that would pass muster using the ISCT standards, whether they do or don’t is actually something that could be addressed by multiple federal agencies as well as consumer protection agencies in the individual states, and, of course, private litigators.
The Product Name
Just the names “StemShot” and “StemVive” are problematic. Any reasonable person would interpret that these are stem cell products solely based on what the company chose to name these 361 tissues.
More Claims
“StemShot® is a minimally-manipulated allograft from donated birth tissue intended for homologous clinical use to repair, reconstruct, replace, or supplement the recipient’s cells or tissues with cell or tissue products performing the same basic function.”
This part is deceiving and counts on the fact that most physicians won’t know that the only homologous use the FDA recognizes for umbilical cord products is hematopoietic reconstitution in cancer care. Given that this e-mail was clearly targeted at an orthopedic and pain management list and not pediatric cancer specialists and given that there is no orthopedic or pain use that the FDA considers homologous, this is saying that you can only use this product for cancer care, but avoiding the details that would reduce the market size.
“StemShot® provides a stem cell-based product meant to supply the extracellular matrix for infiltration, attachment, and proliferation of cells required for the repair and healing of damaged tissue. Medical research has shown such therapies to promote tissue regeneration. StemShot® is the first product to include minimally-manipulated placental material from the membrane, cord tissue, and cord blood and is of the highest quality. The proprietary processing protocols protect and preserve the therapeutic elements from donor tissues and set StemShot® apart from competitors’ products with respect to concentration, bioavailability, and efficacy.”
I have bolded the parts above that are problematic. Claiming that this is a “stem cell product” is a direct violation of the FDCA. Why? The implication is that stem cells in the products are the active ingredient and these cells derive from a donor source, making this a 351 drug product requiring full FDA clinical trials per medical indication and not a quickie 361 45-minute registration.
To dive deeper, under the 21 CFR 1271.10 regulation test, if the product is a “stem cell” product and neither autologous nor used in a close relative, it is a 351 drug product. Also, notice that a claim is made about the product being able to repair tissue and that it is more effective than competitor tissues. Both of these claims can’t be made about a 361 tissue. What can the manufacturers say? They can make claims about what the tissue is or isn’t, how it’s processed, and its basic properties.
Why I Doubt StemShot or Any Other 361 Tissue Has Loads of Viable and Functional Stem Cells
There is a standard series of tests that has existed for a long time in the mesenchymal stem cell (MSC) research world that serve as the minimal requirements for saying you have live and functional cells. These are called the ISCT standards. They provide standardized bars to jump over that all of these amniotic and cord tissue vendors have thus far ignored.
These include…
1. Flow cytometry markers that identify MSCs. Every one of these tests I have seen these cord-tissue companies perform is half-baked and incapable of identifying MSCs. These markers are included and discussed in the video below (no sound):
2. Plastic adherence in culture. The cells must adhere to plastic and form colonies. StemShot claimed to show that they had a colony of stem cells. Below I have included a picture of that colony and what a real stem cell colony from bone marrow looks like: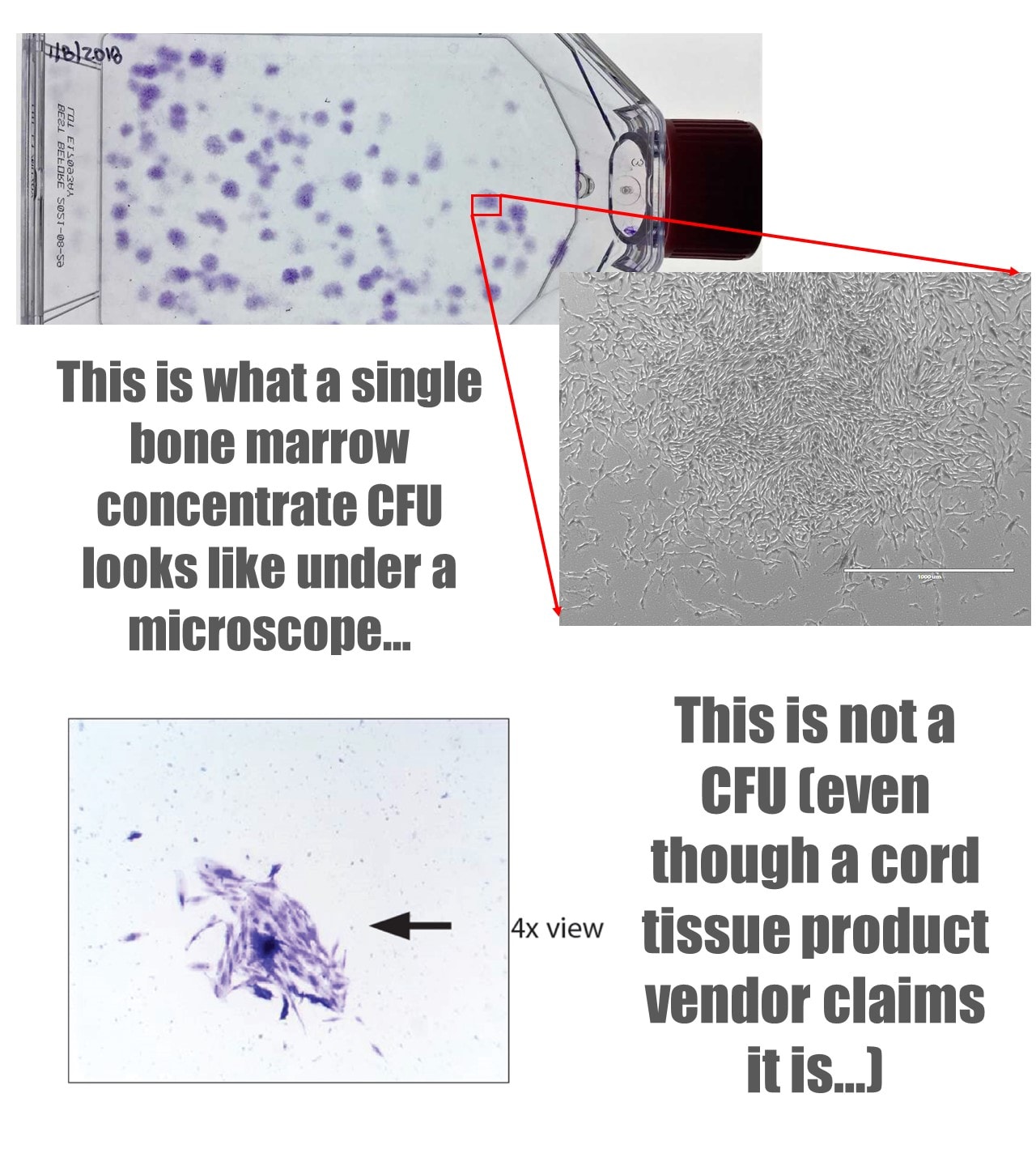
The upshot? I have no idea why an orthopedic sales rep company would want to get involved in assisting an umbilical cord-tissue manufacturer in violating the FDCA. In addition, I have yet to see any company, including the Utah Cord Bank, provide the standard ISCT testing required to make a claim of live and functional mesenchymal stem cells.

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.
