Pluripotent Dead Tissue? CTM Review
If you read this blog, one of my favorite sayings is, “You can’t make this stuff up”. Today let’s review a company that claims to be selling “Pluripotent” birth tissue products that are really dead. In the process, we’ll learn a little about regenerative medicine. Let’s dig in.
What Does “Pluripotent” Mean?
Pluripotent is a regenerative medicine term that most commonly means:
“Pluripotent stem cells are cells that have the capacity to self-renew by dividing and to develop into the three primary germ cell layers of the early embryo and therefore into all cells of the adult body, but not extra-embryonic tissues such as the placenta.”
So the term “pluripotent” means that a stem cell can give rise to the three major germ layers that give rise to all adult cells: the ectoderm, the mesoderm, and the endoderm. Pluripotency or multipotency (cells that can differentiate into fewer cell types) are critical properties of stem cells. Meaning that these cells can develop into many different cells.
Enter CTM Biomedical
CTM biomedical is a company selling placental tissues and that’s located in Palm Beach, Florida. It’s run by a former marketing executive from Skye Biologics, which is a company I have previously covered on this blog. They sell a number of products, but their FDA Tissue Establishment Registration is currently inactive and lists that they were distributing amniotic membrane:
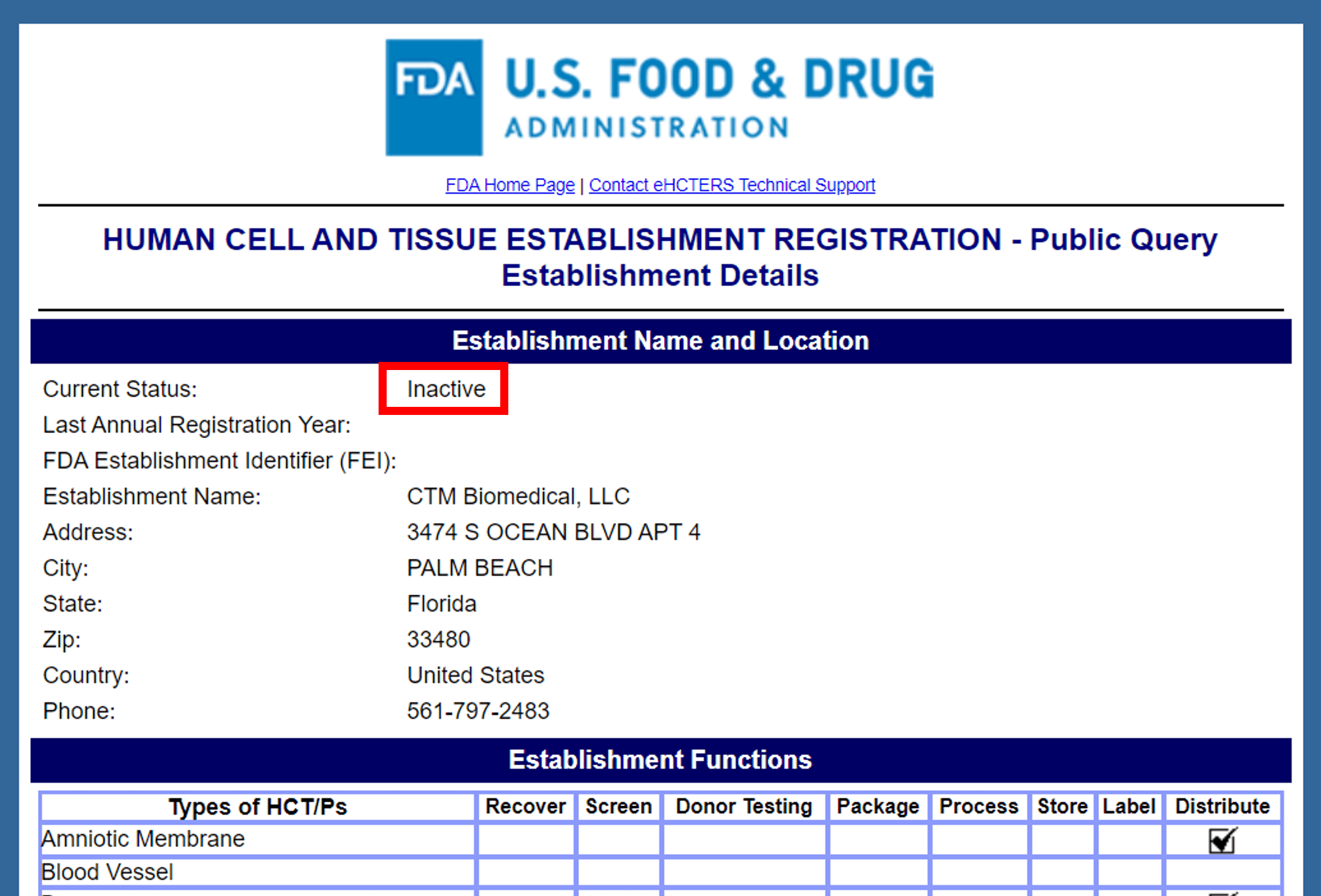
Note that they are NOT listed as a manufacturer, meaning they are a sales rep for someone else’s product. The FDA registration lists the company at a residential apartment building on the beach. The CTM website lists no street address. Other online sources say that the company receives mail at a PO box. I see a handful of salespeople associated with the company on Linkedin, but no other staff.
Slick Minimalist Marketing
I really do like their product marketing (even though they don’t make any products):
Pluripotent Dead Placental Tissues
So far, the CTM story is pretty typical. We have an ex sales executive from another amniotic tissue vendor who has decided to start his own company. CTM doesn’t make their products like many of the placental tissue companies out there. The company is listed in a guy’s apartment in Palm Beach or a PO box and has a handful of sales reps. However, the story takes a slight detour based on an expired FDA tissue registration. However, it takes a careening and abrupt left turn when we see this on the website:
Huh? The tissue sold by CTM Biomedical is stored at room temperature. Hence, by definition, it can have no living cells. Hence, how can it be pluripotent? That would mean that the dead cells and other substances are somehow turning into living cells of a different type? That makes no common sense.
Or does it mean that this extra-cellar matrix (ECM or the stuff that lives between cells) has the ability to turn normal cells into other cells? That brings us to iPSC.
What is iPSC?
Does amniotic membrane induce normal cells to turn into embryonic-like stem cells? When that happens, it’s called induced pluripotency (iPSC means induced pluripotent stem cell). I did find one paper where the amniotic fluid cells from a baby with Hb Bart’s disease could be made into iPS cells (1). You can also take mesenchymal stem cells from living amnion and use the usual reprogramming steps in a lab to get embryonic-like cells (2,3). However, nothing on dead amniotic tissue turning normal cells into embryonic-like iPS cells.
Marketing Hype vs. Reality
The biggest issue in the world of amniotic and placental or umbilical cord products is ridiculous hype. That began with the idea that these products had living, young, and vital stem cells. That bubble was burst by the published tests on these products showing no living cells, let alone stem cells (4-6).
The fact that CTM Biomedical has chosen to call these dead tissue pluripotent is just plain bizarre. That’s taking hype to a new level.
Reality
Placental tissues have some legitimate uses in orthobiologics. We like using them because some have high b-FGF levels and can help PRP help repair damaged tendons. A few early studies are showing promise for some products in helping problems like knee arthritis (7). Hence, there is no reason to hype them, just sell them for what they are.
The upshot? There is no such thing as a pluripotent dead amniotic membrane. As I always say, you can’t make this stuff up.
____________________________________
References:
(1) Yan JM, Li DZ. Generation of Induced Pluripotent Stem Cells from Amniotic Fluid Cells of a Fetus with Hb Bart’s Disease. Hemoglobin. 2017;41(3):198-202. doi:10.1080/03630269.2017.1353523
(2) Slamecka J, McClellan S, Wilk A, et al. Induced pluripotent stem cells derived from human amnion in chemically defined conditions. Cell Cycle. 2018;17(3):330-347. doi:10.1080/15384101.2017.1403690
(3) Guillot PV. Induced pluripotent stem (iPS) cells from human fetal stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:112-120. doi:10.1016/j.bpobgyn.2015.08.007
(4) Berger D, Lyons N, Steinmetz, N. In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics. Interventional Orthopedics Foundation Annual Meeting. Denver, 2015. https://interventionalorthopedics.org/wp-content/uploads/2017/08/AmnioProducts-Poster.pdf
(5) Becktell L, Matuska A, Hon S, Delco M, Cole B, Fortier L. Proteomic analysis and cell viability of nine amnion-derived biologics. Orthopedic Research Society Annual Meeting, New Orleans, 2018. https://app.box.com/s/vcx7uw17gupg9ki06i57lno1tbjmzwaf
(6) Panero, A, Hirahara, A., Andersen, W, Rothenberg J, Fierro, F. Are Amniotic Fluid Products Stem Cell Therapies? A Study of Amniotic Fluid Preparations for Mesenchymal Stem Cells With Bone Marrow Comparison. The American Journal of Sports Medicine, 2019 47(5), 1230–1235. https://doi.org/10.1177/0363546519829034
(7) Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC; ASA Study Group. A Randomized Controlled Single-Blind Study Demonstrating Superiority of Amniotic Suspension Allograft Injection Over Hyaluronic Acid and Saline Control for Modification of Knee Osteoarthritis Symptoms [published correction appears in J Knee Surg. 2019 Nov;32(11):e2]. J Knee Surg. 2019;32(11):1143-1154. doi:10.1055/s-0039-1696672

NOTE: This blog post provides general information to help the reader better understand regenerative medicine, musculoskeletal health, and related subjects. All content provided in this blog, website, or any linked materials, including text, graphics, images, patient profiles, outcomes, and information, are not intended and should not be considered or used as a substitute for medical advice, diagnosis, or treatment. Please always consult with a professional and certified healthcare provider to discuss if a treatment is right for you.



